Control of Chemical Reactions by Hot Electrons Generated at Metal-Oxide Interfaces
Applicable to Energy Conversion and Next-Generation Catalyst Development, with Potential for Greenhouse Gas Reduction
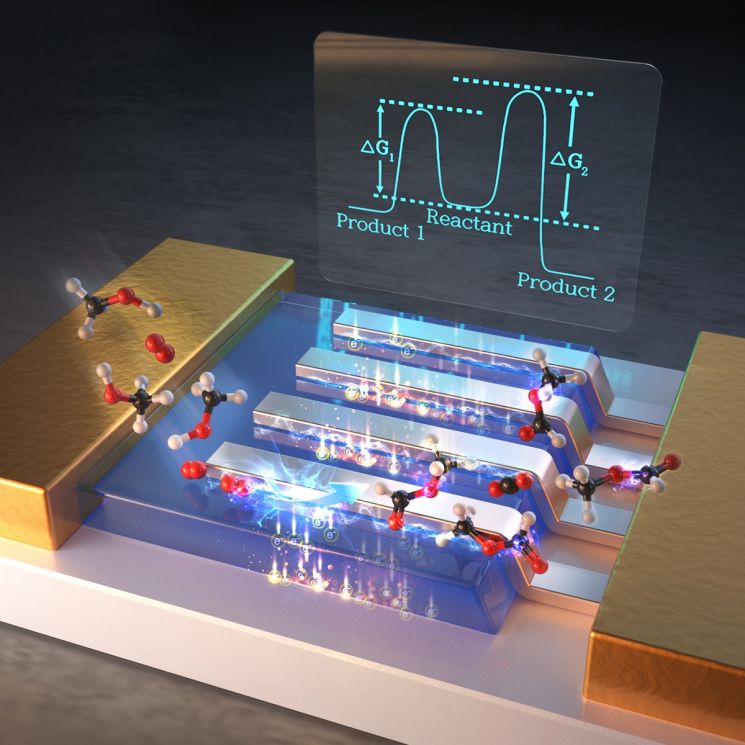 Schematic diagram of a hot electron catalytic device fabricated by depositing platinum nanowires on titania and the principle of greenhouse gas reduction using it
Schematic diagram of a hot electron catalytic device fabricated by depositing platinum nanowires on titania and the principle of greenhouse gas reduction using it
[Asia Economy Reporter Junho Hwang] A method to improve the efficiency of selective oxidation of alcohols during chemical catalytic reactions has been developed. This partial oxidation process enables the production of industrially valuable chemical fuels such as formaldehyde and formic acid while reducing carbon dioxide emissions.
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 8th that a joint research result by Professor Jung-Young Park of the Department of Chemistry (Deputy Director of the Institute for Basic Science Center for Nanomaterials and Chemical Reactions), Professor Yeon-Sik Jeong of the Department of Materials Science and Engineering, and Professor Yoo-Sung Jung of the Department of Bio and Chemical Engineering was recently published in the international journal Nature Communications.
The research team elucidated the mechanism of catalytic chemical reactions at the metal-oxide interface and demonstrated that hot electrons are a decisive factor in enhancing catalytic selectivity. Hot electrons refer to an energized state during the instantaneous conversion of chemical energy when external energy such as molecular adsorption, chemical catalytic reactions, or light absorption is transferred to the metal surface.
First, the team developed a novel catalyst by attaching platinum nanowires to titanium oxide to control the 'metal nanowire-oxide interface.' Using a hot electron catalytic device based on this interface, they observed the movement of hot electrons in real time. Through this, they confirmed that when the metal-oxide interface is formed, the production efficiency of methyl formate?formed by the partial oxidation of methanol?increases while carbon dioxide generation decreases. Methyl formate serves as a raw material for producing formaldehyde and formic acid.
The team also identified that the high selectivity of this catalyst is related to the amplified generation of hot electrons at the interface formed on the catalyst surface. In particular, through comparative quantum mechanical modeling calculations, the team theoretically proved that the amplified catalytic selectivity at the metal nanowire-oxide interface originates from a completely different catalytic reaction mechanism at the interface.
 Detection of Hot Electron Transfer Generated by Catalytic Reactions at Metal-Oxide Interfaces Using Hot Electron Catalytic Devices Fabricated by Depositing Platinum Nanowires on Titania
Detection of Hot Electron Transfer Generated by Catalytic Reactions at Metal-Oxide Interfaces Using Hot Electron Catalytic Devices Fabricated by Depositing Platinum Nanowires on Titania
Professor Jung-Young Park, who led the research, said, "Using hot electrons and the metal nanowire-oxide interface, it is possible to reduce the generation of the greenhouse gas carbon dioxide and increase the production of high value-added chemical fuels. The concept that catalytic selectivity can be controlled through hot electrons and the metal-oxide interface can be applied to energy conversion and next-generation catalyst development, and is expected to have applications such as reducing greenhouse gases, the main cause of global warming."
Professor Yeon-Sik Jeong of the Department of Materials Science and Engineering, who led the research on the junction of nanowires and oxides, stated, "This study made it possible to detect hot electrons at the metal-oxide interface, which was technically difficult in existing catalytic device systems, thanks to very precise nanowire printing technology. We expect this technology to be utilized in the development of various next-generation hybrid catalysts in the future."
Professor Yoo-Sung Jung of the Department of Bio and Chemical Engineering, who led the theoretical calculations proving the relationship between the interface and catalytic selectivity, also explained, "This research experimentally observed and theoretically demonstrated through quantum calculations that the metal-oxide interface can play an important role in enhancing selectivity in catalytic chemical reactions. It is expected to be utilized in the development of chemical processes using heterogeneous catalysts."
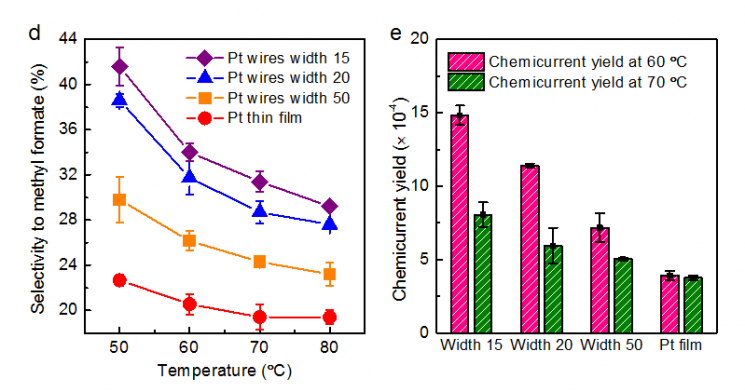 Changes in catalytic selectivity and hot electron detection efficiency according to the linewidth of platinum nanowires (concentration at the metal-oxide interface) in the developed hot electron catalytic device
Changes in catalytic selectivity and hot electron detection efficiency according to the linewidth of platinum nanowires (concentration at the metal-oxide interface) in the developed hot electron catalytic device
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















