Discovery of Intracellular Transport-Related 'RAN Protein' Distribution Control Pathway
Normalization of RAN Distribution and Inhibition of Neuronal Cell Death via LSM12-EPAC1 Pathway
[Asia Economy Reporter Junho Hwang] Domestic researchers have discovered a neuroprotective gene that suppresses proteins causing degenerative brain diseases such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. On the 28th, Ulsan National Institute of Science and Technology (UNIST) announced that Professor Jeonghoon Lim's research team in the Department of Life Sciences identified the LSM12 and EPAC1 genes and elucidated their neuroprotective pathways. This research was recently published in the international journal PLOS Biology.
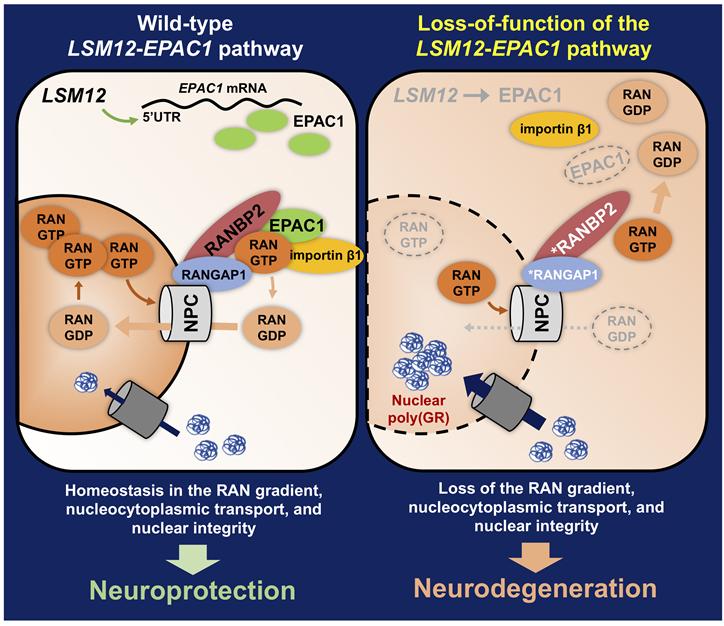
Material transport between the cell nucleus and the surrounding cytoplasm is essential for maintaining cellular function. Impairment of nucleus-cytoplasm material transport has recently been identified as one of the causes of neuronal cell death in various degenerative brain diseases.
The genes discovered by the research team correct the abnormal distribution of a specific protein (RAN protein) inside the neurons of ALS patients, thereby suppressing cell death. Generally, RAN protein is more abundant inside the nucleus, but in ALS patients, RAN protein leaks into the cytoplasm, causing an abnormal concentration gradient. The LSM12-EPAC1 gene pathway discovered by the team normalizes the RAN protein concentration gradient that determines the direction of nucleus-cytoplasm material transport, restoring cellular function.
Jongbo Lee, a first author and integrated master's and doctoral student in the Department of Life Sciences at UNIST, who participated in the study, explained, "To verify the neuroprotective effect of the discovered gene pathway, we used neurons differentiated from induced pluripotent stem cells of ALS patients. By activating this gene pathway, we suppressed the abnormal distribution of RAN protein and cytotoxicity observed in neurons of ALS patients."
Additionally, the research team elucidated the detailed mechanism by which the EPAC1 protein, expressed through the LSM12-EPAC1 gene pathway, regulates RAN protein distribution. EPAC1 protein enhances the binding affinity between the nuclear pore complex and RAN protein. As a result, RAN protein that has leaked into the cytoplasm is easily captured by the nuclear pore complex and sent back inside the nucleus. The nuclear pore complex is a massive protein assembly embedded in the nuclear membrane (the membrane surrounding the nucleus) that serves as a passage for material exchange between the nucleus and cytoplasm.
Professor Jeonghoon Lim stated, "Although it was known that the distribution of RAN protein within cells is important for nucleus-cytoplasm material transport, the molecular biological regulatory mechanisms were not well understood. This study revealed that two previously unrelated genes form a gene pathway that regulates the distribution of RAN protein within cells."
Professor Lim added, "This research is expected to make significant contributions not only to the prediction and treatment of related diseases such as ALS and frontotemporal dementia but also to establishing foundational knowledge for the molecular biological understanding of the general aging process."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.