Secured 44 Million COVID-19 Vaccine Doses
Global Joint Purchase and Individual Company Negotiations
Free Vaccination for Virtually the Entire Population Except Children Expected
[Asia Economy Reporters Seo So-jeong and Choi Dae-yeol] Although the government has secured COVID-19 vaccines for 44 million people, there are many preliminary tasks to be completed before the vaccines can be administered to the public, such as distribution systems and safety verification. The characteristics of the vaccines pre-purchased by the government?AstraZeneca, Pfizer, Janssen, and Moderna?are all different, as are their storage conditions and the number of doses required. In particular, since vaccine development is not yet complete, concerns about safety and efficacy have been raised, making it necessary to monitor foreign trends and side effects.
① Pfizer Vaccine Cold Chain Is Key
Each Vaccine Has Different Distribution Considerations
Especially for the Pfizer vaccine, which began administration in the UK on the 8th (local time), strict preparation is required due to the demanding cold chain conditions, such as storage at minus 70 degrees Celsius. The Pfizer vaccine can only be stored for ten days in special containers filled with dry ice, and separate freezers capable of consistently maintaining temperatures below minus 70 degrees Celsius must be available.
From the logistics process of transporting the vaccine from Incheon International Airport to medical sites to ensuring safe delivery to storage facilities at each hospital, a thorough plan for the entire distribution system must be meticulously prepared. Lim In-taek, Director of the Health Industry Policy Bureau at the Ministry of Health and Welfare, said, "The Pfizer vaccine requires two doses, not just one, and ultra-low temperature distribution is necessary, so the government is preparing thorough countermeasures. We are discussing distribution and storage extensively with the Ministry of Food and Drug Safety and gathering expert opinions to ensure complete vaccination."
To this end, a dedicated task force (COVID-19 Vaccination Response Promotion Team) will be established within the Korea Disease Control and Prevention Agency for vaccine procurement and immunization.
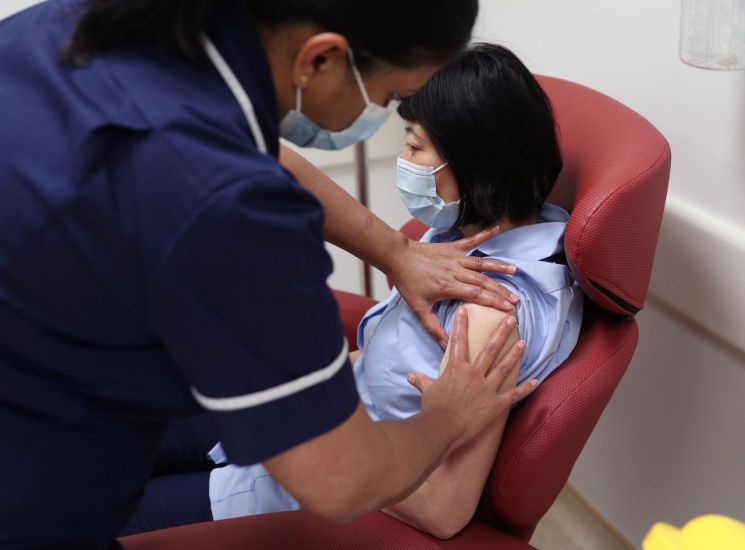 A nurse at a hospital in London, England, is conducting a simulation training for the COVID-19 vaccine developed by the American pharmaceutical company Pfizer. <이미지:연합뉴스>
A nurse at a hospital in London, England, is conducting a simulation training for the COVID-19 vaccine developed by the American pharmaceutical company Pfizer. <이미지:연합뉴스>
② Safety Concerns Including Side Effects
Manufacturers Demand No Liability
Safety concerns, including side effects, have not been completely resolved. Vaccines widely used for influenza and other diseases have been developed over several years to decades and have been administered over long periods, proving their safety. COVID-19 vaccines, however, have been developed in less than a year, skipping lengthy clinical trials, leaving potential side effect concerns. Vaccine manufacturers have requested not to be held liable for side effects after vaccination during negotiations with each country. Although the government has not disclosed specific contract details, considering this common request, it is expected that South Korea is in a similar position.
Accordingly, health authorities are coordinating the timing of vaccination to observe trends in countries that have started vaccination earlier rather than beginning immediately upon vaccine introduction domestically. It is likely to be conducted as a national immunization program similar to influenza vaccination, which requires establishing compensation measures for side effects after vaccination.
For the vaccines secured this time, clinical trial data for children are unavailable, so children are expected to be excluded from the vaccination target group due to concerns about side effects. Typically, short-term side effects can be identified within 2 to 3 months, while long-term side effects may take years to manifest. Yang Dong-gyo, Director of the Medical Safety Prevention Bureau at the Korea Disease Control and Prevention Agency, stated, "During the process of adjusting vaccination targets and timing, we plan to establish a detailed compensation system for vaccine side effects in accordance with the Infectious Disease Control and Prevention Act."
There is also a possibility that vaccines still under development may ultimately fail. The AstraZeneca and Janssen vaccines secured by our government are in the final stages of clinical trials. In the case of pre-purchase contracts, it is common that even if the vaccine ultimately fails, advance payments and other amounts already paid cannot be refunded.
 Professor Emeritus Hwanjong Lee of Seoul National University College of Medicine is answering questions at the briefing on the COVID-19 vaccine introduction plan held on the morning of the 8th at the briefing room of the Government Seoul Office Annex in Jongno-gu, Seoul. Photo by Hyunmin Kim kimhyun81@
Professor Emeritus Hwanjong Lee of Seoul National University College of Medicine is answering questions at the briefing on the COVID-19 vaccine introduction plan held on the morning of the 8th at the briefing room of the Government Seoul Office Annex in Jongno-gu, Seoul. Photo by Hyunmin Kim kimhyun81@
③ Introduction Early Next Year, Vaccination in Second Half
Preparation to Avoid Approval Delays
Advancing the vaccination schedule is also a challenge. The government plans to gradually introduce the pre-purchased vaccines starting in the first quarter of next year (February-March), with public vaccination expected in the second half of next year. Yang Jin-young, Deputy Director of the Ministry of Food and Drug Safety, said, "Unlike general pharmaceuticals, vaccines require national batch release approval. We have formed a dedicated rapid approval review team and are preparing accordingly. We will coordinate with the Ministry of Health and Welfare and the Korea Disease Control and Prevention Agency to ensure there are no disruptions to the vaccination schedule."
The AstraZeneca vaccine will be introduced first in the first quarter of next year, followed sequentially by Pfizer, Moderna, and other vaccines. However, as the number of confirmed cases continues to surge in countries such as the United States, the United Kingdom, and the European Union (EU), these countries are likely to receive priority supply. Director Lim said, "The COVID-19 situation in the United States is very urgent, so supply to those countries is likely to be prioritized, but South Korea will not be delayed compared to countries like Japan. We will make every effort to ensure timely introduction and vaccination."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















