"Safety and Efficacy Clearly Verified"
[Asia Economy Reporter Cho Hyun-ui] Domestic novel coronavirus disease (COVID-19) vaccines are expected to be released at the earliest in the second half of next year. Although the development speed is slower than overseas vaccines that are already in the final stages of release, the strategy is to fully secure safety and efficacy.
According to the industry on the 8th, Genexine, SK Bioscience, GeneOne Life Science, Cellid, and iGEN are developing domestic COVID-19 vaccines. Cellid's 'adCLD-CoV19' received approval for clinical phase 1 and 2 trial plans (IND) from the Ministry of Food and Drug Safety on the 4th. Cellid's vaccine is a viral vector vaccine manufactured by inserting the surface antigen gene of the COVID-19 virus into an adenovirus template. The UK’s AstraZeneca, the US’s Johnson & Johnson, Russia’s Gamaleya Research Institute, and China’s CanSino are also developing COVID-19 vaccines using this method.
GeneOne Life Science's GLS-5310 also received approval for clinical phase 1 and 2 trial plans on the 4th. This vaccine is a DNA vaccine manufactured in the form of DNA (plasmid) containing the surface antigen gene of the COVID-19 virus. Cellid and GeneOne Life Science will evaluate the safety and immunogenicity of these vaccines in healthy adults during clinical phases 1 and 2.
SK Bioscience received approval for the clinical phase 1 trial plan (IND) of its self-developed 'NBP2001' from the Ministry of Food and Drug Safety on the 23rd of last month and has entered clinical trials. NBP2001 is a protein recombinant vaccine. SK Bioscience stated, "Since it is a stabilized synthetic antigen vaccine produced through protein culture and purification processes, it is expected to be advantageous in securing high safety even in clinical phase 1."
Along with this, SK Bioscience is conducting non-clinical trials of the COVID-19 vaccine candidate GBP510 with support from the Bill & Melinda Gates Foundation. Unlike NBP2001, GBP510 is known to utilize a different platform rather than a synthetic antigen vaccine. SK Bioscience aims to apply for clinical phase 1 of GBP510 to the Ministry of Food and Drug Safety within this month and enter clinical phase 1 within the year. Ahn Jae-yong, CEO of SK Bioscience, stated, "Our goal is to develop a COVID-19 vaccine that is somewhat later than the first vaccines released globally but has thoroughly verified safety and efficacy."
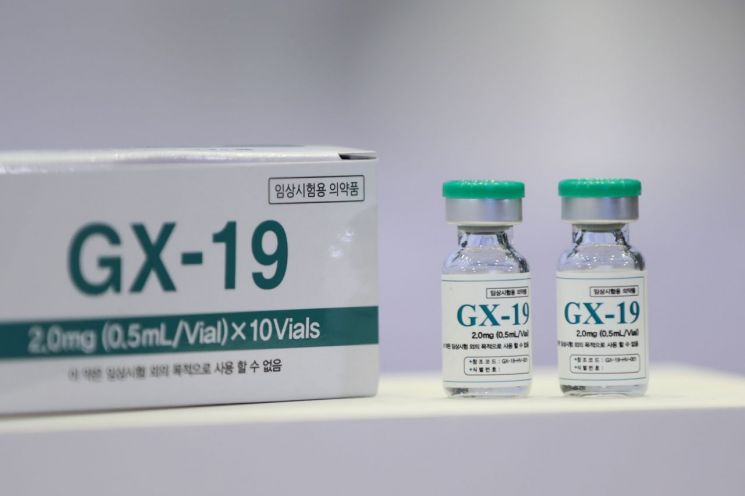
Genexine’s GX-19, which is developing a DNA vaccine, is in the final stage of clinical phase 1. Based on the results derived from clinical phase 1, Genexine plans to enter clinical phase 2 by the end of this month at the latest. Genexine, which received 9.3 billion KRW in funding from the government, plans to conduct clinical phase 3 as early as the first quarter of next year. Sung Young-chul, Chairman of Genexine, said, "We plan to conduct clinical phases 2b and 3 simultaneously in the first half of next year and expect to apply for marketing approval from the Ministry of Food and Drug Safety by September next year."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














