Obstacle to All-Solid-State Battery Commercialization
Establishment of Electrolyte-Electrode Interface Resistance Analysis Platform
Foundation for Material Design Enhancing Performance and Output Through Cathode Material Self-Improvement
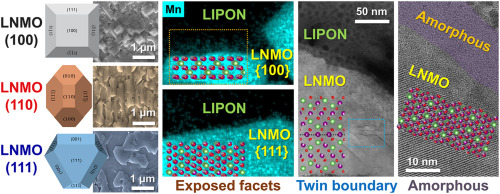 Schematic diagram and photo of epitaxially grown LiNi0.5Mn1.5O4 (LNMO) cathode exposed crystal planes, controlling the exposed crystal planes to (100), (110), and (111) planes
Schematic diagram and photo of epitaxially grown LiNi0.5Mn1.5O4 (LNMO) cathode exposed crystal planes, controlling the exposed crystal planes to (100), (110), and (111) planes
[Asia Economy Reporter Hwang Junho] Domestic researchers have devised a solution to the chronic problems of all-solid-state batteries, which are considered a replacement for lithium-ion batteries used in electric vehicles. The commercialization of all-solid-state batteries has been delayed due to increased interfacial resistance between the solid electrolyte and the cathode, which can cause fires. The researchers have now found a solution to this issue.
The research team led by Dr. Park Sang-baek from the Energy Materials Research Group at the Korea Institute of Science and Technology (KIST) and the team led by Professor Shin Hyun-jung from Sungkyunkwan University announced on the 29th that they identified a method to reduce interfacial resistance between cathodes by analyzing the crystal structure of the materials. Their research results were recently published in the international journal Nano Energy.
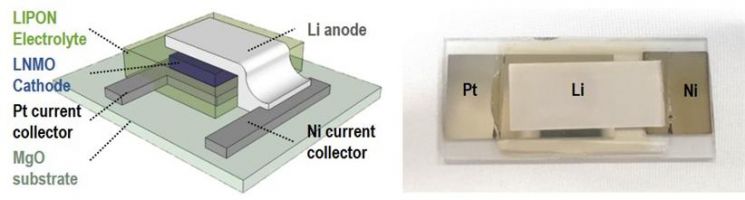 Schematic and photo of an epitaxially grown LiNi0.5Mn1.5O4 (LNMO) thin-film-based all-solid-state battery
Schematic and photo of an epitaxially grown LiNi0.5Mn1.5O4 (LNMO) thin-film-based all-solid-state battery
In all-solid-state batteries with solid electrode-solid electrolyte interfaces, limited charge transfer and disruption of atomic arrangements cause resistance and degradation. To address this, the research team examined the crystal structure at the interface where the solid electrolyte meets the cathode.
Using epitaxial thin-film technology?a semiconductor manufacturing technique that grows thin films along the direction of the substrate’s crystal formation?they obtained cathode thin films with various exposed crystal planes of particles. They then excluded factors such as particle size and contact area to meticulously analyze the effect of the exposed crystal planes on the interface between the solid electrolyte and cathode materials.
As a result, they confirmed that when the crystal surface within atoms is densely packed, the leakage of transition metals, which should remain within the cathode material, into the electrolyte is suppressed, thereby improving the stability of all-solid-state batteries. They also discovered that when the boundaries of crystals are aligned parallel to the direction of electron movement, ions and electrons moving along the crystals are not hindered, reducing resistance and increasing output.
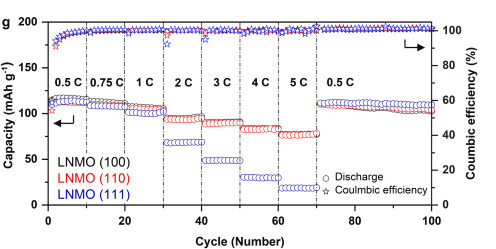 Changes in battery performance according to exposure decision surfaces (100, 110, 111) show that over time, the 111 surface demonstrates superior performance.
Changes in battery performance according to exposure decision surfaces (100, 110, 111) show that over time, the 111 surface demonstrates superior performance.
Dr. Park Sang-baek stated, "Increasing the density of the crystal surface and controlling the orientation of crystal boundaries means that high performance and stability can be secured by improving the cathode material itself." He added, "Based on this research that elucidates the mechanism of performance degradation in all-solid-state batteries, we plan to accelerate the development of all-solid-state battery materials that overcome the instability at the solid electrolyte-cathode solid interface and provide high ion-charge exchange characteristics."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















