Professor Jang Jihyun's Team Increases Supercapacitor Capacity Using Perovskite Oxide Electrode Material
Published Online in ACS Energy Letters on November 13
Ulsan National Institute of Science and Technology Accelerates Development of High-Speed C
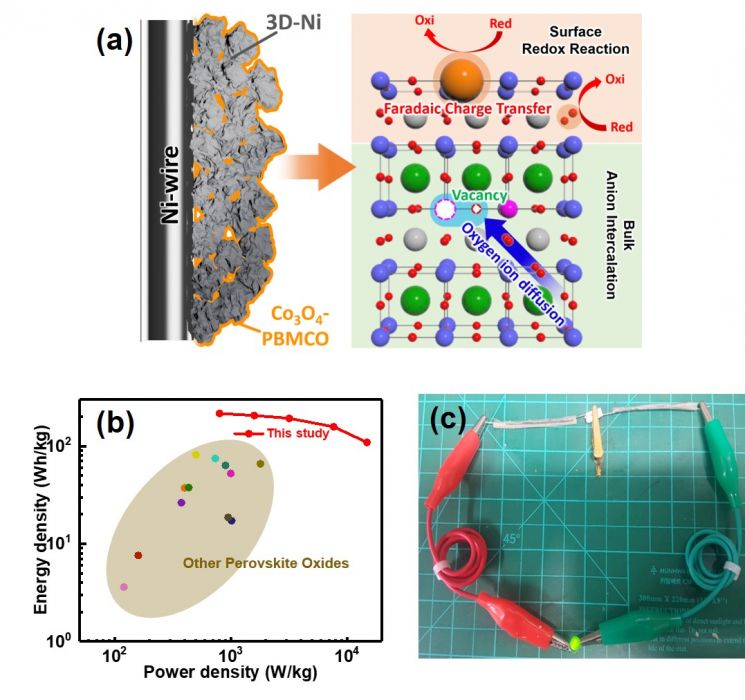 Figure of the principle of electrical energy storage of the developed material and performance study of the hybrid supercapacitor applied with it.
Figure of the principle of electrical energy storage of the developed material and performance study of the hybrid supercapacitor applied with it.
[Asia Economy Yeongnam Reporting Headquarters, Reporter Kim Yong-woo] Is it possible to store items on the walls, fences, and ceiling of a warehouse?
A storage material that uses both the inside and the shell of a substance has emerged. Since even the space occupied by the surface can be used as storage, the capacity increases accordingly.
A perovskite material capable of storing energy both on the surface and inside the substance has been developed.
The research team led by Professor Jang Ji-hyun of the Department of Energy Chemical Engineering at Ulsan National Institute of Science and Technology (UNIST, President Lee Yong-hoon) developed a perovskite oxide material that can be used in a new concept energy storage device combining the advantages of secondary batteries and supercapacitors (large-capacity electrical storage devices).
They succeeded in manufacturing a flexible supercapacitor for wearable device power sources by simply coating this material on electrodes, opening the path to commercialization.
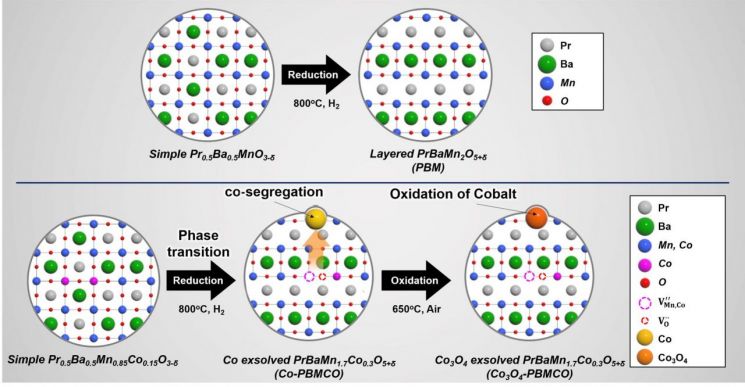 Schematic diagram of the synthesis of double-layered perovskite oxide with eluted transition metals.
Schematic diagram of the synthesis of double-layered perovskite oxide with eluted transition metals.
Consequently, the development of a versatile battery (energy storage device) with fast charging and high output that combines the large capacity of secondary batteries with the advantages of supercapacitors is expected to be accelerated.
Unlike secondary batteries, supercapacitors are power devices that charge quickly and can rapidly supply electricity when needed. They store electrical energy on the electrode 'surface' and then release it.
They also have an almost permanent lifespan and are lightweight. They can be made very small, attracting attention as power sources for the Internet of Things and wearable devices.
However, they have the limitation of lower energy storage capacity per unit mass compared to secondary batteries like lithium-ion batteries, which store electricity inside the material.
The research team enhanced the energy storage capacity of supercapacitors by using perovskite oxide-based electrode active materials that can store energy both on the surface and inside the material.
This means employing both the energy storage methods of supercapacitors and secondary batteries.
The oxygen anions inside the material play a role similar to lithium cations in secondary batteries, storing electrical energy inside the material, while cobalt (Co) exsolved from inside undergoes oxidation and stores electrical energy on the surface in the supercapacitor manner.
Kang Kyung-nam, a doctoral researcher in the Department of Chemical Engineering at UNIST and first author of the study, explained, “We developed a new material based on the observation that cobalt in the perovskite oxide exsolves to the surface and that there are many oxygen vacancies?empty spaces where oxygen anions can freely move?within the perovskite oxide.”
The flexible supercapacitor coated with this material on the electrode recorded an energy density per unit mass (energy storage capacity of a single electrode) of 215.8 Wh/kg (218.54 mAh/g).
This is about a 60% improvement compared to when conventional perovskite materials were applied. Additionally, the power density, an indicator of instantaneous output, was high at 14.8 kW/kg.
Using this supercapacitor, a 3.6V LED light could be powered, and it maintained stable performance even when bent or twisted.
Professor Jang Ji-hyun said, “This research proved that every part of the material can be used for energy storage,” adding, “Through this, we complemented the limitations of existing secondary batteries and supercapacitors and selectively combined their advantages, presenting a new direction for developing next-generation energy storage devices.”
Professor Jang also stated, “We will continue research not only on utilizing perovskite oxides as electrode active materials for next-generation batteries but also on developing new electrode materials that can apply this new concept of energy storage.”
The study also involved Professor Kim Geon-tae from the Department of Energy Chemical Engineering at UNIST and Professor Jeong Hu-young from the Research Support Headquarters. The research results were selected as the front supplementary cover paper in the international energy journal ACS Energy Letters and published online on November 13.
The research was supported by the Korea Research Foundation (NRF) mid-career researcher support project and the onsite hydrogen refueling station photoelectrochemical hydrogen production technology and system development project.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.



















