Promoting Water Electrolysis with Low-Cost Metals
Expected to Reduce Hydrogen Production Costs
[Asia Economy Reporter Junho Hwang] A technology has been developed that can convert gray hydrogen into green hydrogen. This production method costs only one-twentieth of the existing hydrogen production cost, has six times higher hydrogen productivity, and four times longer sustainability. It is expected to be a technology that will contribute to establishing hydrogen as an eco-friendly future fuel.
The research team led by Associate Director Hyoyoung Lee of the Nano-Structure Physics Research Group at the Institute for Basic Science announced on the 24th that they have developed a water-splitting catalyst capable of producing hydrogen in an eco-friendly manner, and the related research paper was published in the international academic journal Energy & Environmental Science (IF 30.287).
Converting Gray Hydrogen into Green Hydrogen
Hydrogen fuel is attracting attention as an eco-friendly future fuel that can replace fossil fuels. However, in reality, it is produced during the oil refining process, which generates carbon dioxide. Therefore, hydrogen fuel is commonly called "gray hydrogen."
Electrolysis, which obtains hydrogen by splitting water, is the only production method that does not generate carbon dioxide, but it has the disadvantage of low productivity due to the slow oxygen evolution reaction. To increase production speed, ruthenium oxide (RuO2) and iridium oxide (IrO2) can be used, but their price exceeds $70,000 per kilogram, making them economically unfeasible. It is also difficult to produce hydrogen continuously for more than 24 hours.
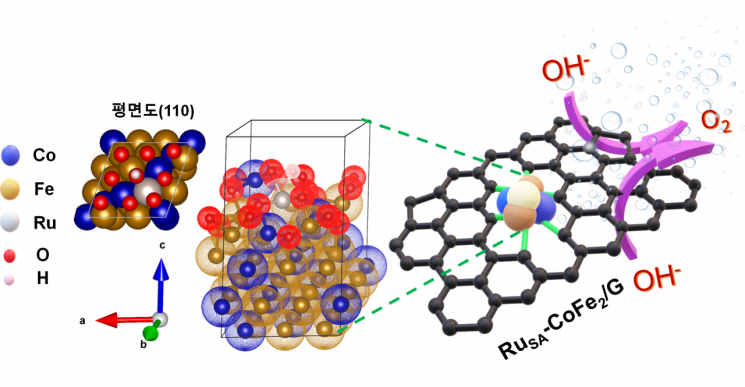
The research team developed a water electrolysis catalyst by attaching oxygen atoms onto inexpensive transition metals such as cobalt, iron, and a very small amount of ruthenium. To accelerate the electrolysis reaction rate, oxygen must be attached to the catalyst surface, for which a cobalt-iron alloy was utilized. Then, a small amount of ruthenium was added to further increase the oxygen reaction rate.
Producing More Hydrogen with Affordable Catalysts
The research team stated that they were able to produce six times more hydrogen at one-twentieth the cost of existing catalysts and achieved a reaction that lasted at least 100 hours. In particular, they emphasized that the oxygen reaction occurred at a much lower voltage than electrolysis using conventional ruthenium oxide catalysts.
Generally, the faster the oxygen evolution rate, the higher the current density. The conventional ruthenium oxide (RuO2) catalyst requires 298 millivolts (mV) to achieve a current density of 10 milliamperes per square centimeter (mA/cm2). However, the electrocatalyst developed by the research team achieved a similar effect with only 180 millivolts of current density.
Associate Director Hyoyoung Lee said, “Producing eco-friendly hydrogen through water splitting at a price cheaper than petroleum- and coal-derived hydrogen has long faced limitations.” He added, “By developing an inexpensive and highly efficient oxygen evolution catalyst, we expect to take a step closer to a decarbonized, eco-friendly hydrogen economy.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










