MFDS: "Imported through import approval... No clinical plans at all"
 Fake news claiming that multinational pharmaceutical company AstraZeneca is recruiting participants for a COVID-19 vaccine clinical trial
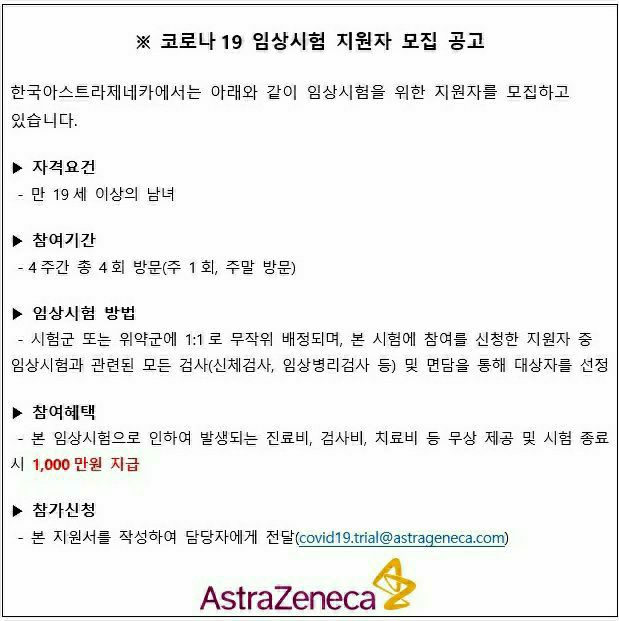
Fake news claiming that multinational pharmaceutical company AstraZeneca is recruiting participants for a COVID-19 vaccine clinical trial
[Asia Economy Reporter Cho Hyun-ui] As the Ministry of Food and Drug Safety (MFDS) has begun expedited approval for the novel coronavirus disease (COVID-19) vaccine developed by the multinational pharmaceutical company AstraZeneca, there are concerns about fake news circulating that claims "recruitment of domestic clinical trial volunteers." The MFDS emphasized on the 29th that "there are absolutely no plans to conduct clinical trials domestically."
On the previous day, after the MFDS announced the formation of a dedicated review team for AstraZeneca's vaccine approval, an image in the form of an official letter recruiting volunteers for the Korea AstraZeneca COVID-19 clinical trial spread mainly through internet communities and group chat rooms.
The image stated, "Men and women aged 19 and over who visit once every weekend for 4 weeks, totaling 4 visits, will be provided free medical fees, examination fees, and treatment fees related to the clinical trial, and 10 million KRW will be paid after the trial ends."
Regarding the clinical trial method, it said, "Participants will be randomly assigned one-to-one to either the test group or the placebo group, and candidates will be selected through all clinical trial-related examinations (physical examination, clinical pathology tests, etc.) and interviews among the applicants."
The MFDS drew a clear line, calling this "fake news." An MFDS official told Asia Economy in a phone interview, "Korea AstraZeneca has never submitted an Investigational New Drug (IND) application to the MFDS." To conduct clinical trials domestically, pharmaceutical companies and research institutions must apply for an IND to the MFDS, which must approve it.
The MFDS especially stated that since the plan is to introduce AstraZeneca's vaccine by importing it, there are absolutely no plans to conduct clinical trials domestically. An MFDS official said, "We are coordinating to approve import based on clinical trials and approval data conducted overseas without conducting separate clinical trials domestically," adding, "Currently, there are absolutely no plans to conduct clinical trials in Korea."
Recently, amid reports that a 28-year-old man who participated in the phase 3 clinical trial of this vaccine in Brazil died, this fake news attracted significant attention. In particular, the domain of the contact email listed in the image (astrageneca.com) differed from the actual Korea AstraZeneca domain (astrazeneca.co.kr), causing visitors attempting to verify authenticity to temporarily overload the Korea AstraZeneca website the previous day.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















