[Asia Economy Reporter Seo So-jeong] The fees that pharmaceutical companies and others must pay to the Ministry of Food and Drug Safety for drug approval reviews will increase by up to 30%.
The Ministry of Food and Drug Safety has revised the "Fee Regulations on the Approval of Drugs, etc." (Ministry of Food and Drug Safety Notice), which includes a major increase in approval fees for drugs to strengthen expertise in the field of drug approval and review, and will implement it from the 23rd.
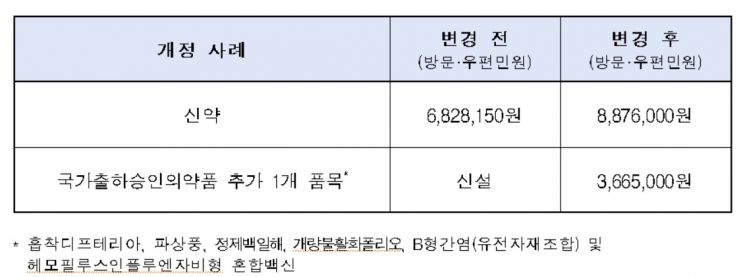
Accordingly, the new drug approval fee, which was 6.82 million KRW in 2016, will rise to 8.87 million KRW this year.
This revision is the first in four years since 2016. It was promoted to improve drug approval review work by securing review personnel through fee rationalization.
The main revisions include ▲ approximately 30% increase in drug approval fees ▲ addition of items for national batch release approval drugs.
The Ministry of Food and Drug Safety stated, "Through this increase in fees for drugs, we will expand specialized personnel for approval reviews to enhance expertise, and strive to promptly approve drugs that ensure safety and quality through meticulous review and evaluation."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















