Trace Metal Ions in Enzymes Reveal Secrets of Enzyme Activity
Binding-Induced Stereochemical Changes Regulate Enzyme Activity Levels
Controlling Enzyme Activity Enables New Drug Development
[Asia Economy Reporter Junho Hwang] Why does the performance of an enzyme, which is a protein cluster, drop sharply when metal ions are removed from it?
A research team led by Professors Cheolmin Kim and Chaewoon Kim from the Department of Physics at Ulsan National Institute of Science and Technology revealed through years of research that metal ions within enzymes regulate enzyme activity. Their research paper was recently published in the international journal Nature Communications. On the 19th, the research team anticipated that by elucidating the role of metal ions, it would be possible to regulate enzyme activity and even develop new drugs.
Metal ions regulate enzyme reactions
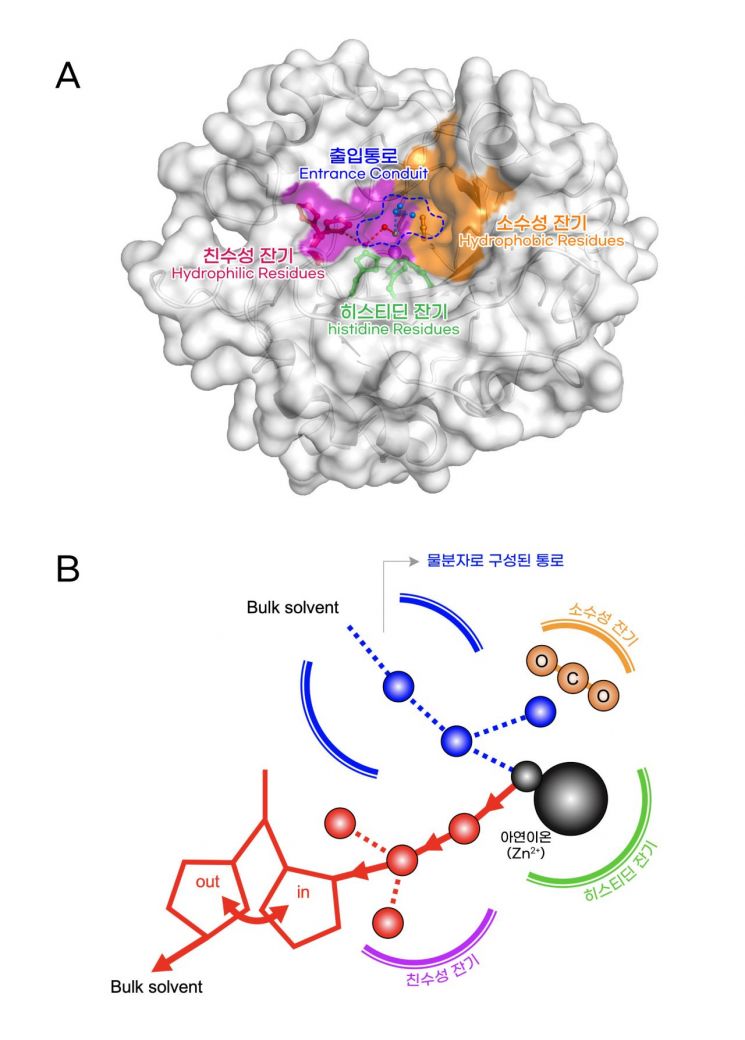
Carbonic anhydrase is an enzyme that helps convert carbon dioxide (reactant) into a form of carbonic acid (product) that dissolves well in blood. In its natural state, this enzyme contains zinc ions. When these zinc ions are replaced with other metal ions that have similar chemical properties, the enzyme's performance (activity) drops sharply. However, the reason for this phenomenon had not been clarified.
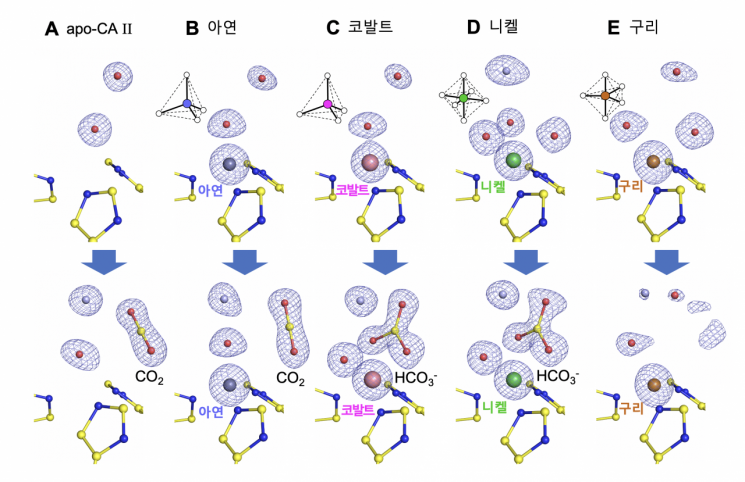
The research team revealed through this study that the three-dimensional geometric structure (coordination geometry) around the metal ion determines the degree of enzyme activity.
This structure regulates enzyme activity in two main ways. First, the spatial structure determines the rate at which the reactant (carbon dioxide) attaches to the enzyme and the product (carbonic acid) detaches from the enzyme. For high enzyme activity, the reactant must attach well to the active site of the enzyme, and the product must detach easily. Second, the spatial structure delicately controls the structure and arrangement of surrounding water molecules, which also affects enzyme activity. Water molecules present within the enzyme either directly participate in the reaction converting reactants to products or form the passage through which reactants and products travel.
Contributing to new drug development by regulating enzyme reactions
The research team obtained these results by capturing the brief moment when enzyme reactions occur using a high-pressure rapid cooling technique. This technique was developed by Professor Chaewoon Kim’s team in previous research. By injecting carbon dioxide (reactant) gas into the enzyme and then rapidly cooling it, the moment of reaction can be captured. The team prepared enzyme crystals containing four types of metal ions and enzyme crystals without metal ions using this technique and analyzed them using X-ray crystallography.
Jinkyun Kim, the first author and a graduate student in the integrated master’s and doctoral program in the Department of Natural Sciences, said, "This research is the result of years of persistent efforts to capture the intermediate stages of enzyme reactions."
Professor Chaewoon Kim stated, "The significance of this study lies in discovering that trace amounts of metal ions within enzymes act as conductors overseeing enzyme activity through broad and delicate interactions."
Professor Cheolmin Kim explained, "If the role of metal ions is limited to Lewis acids, it cannot explain the phenomenon where enzyme activity decreases when copper ions or cobalt ions with the same 'valence electron count' are used as cofactors. Through this study, we identified another role of metal cofactors."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.