[Asia Economy Reporter Junho Hwang] An industrial catalyst mimicking enzymes that act as catalysts in our bodies has been developed. Just as enzymes are surrounded by soft organic polymers to selectively induce catalytic reactions, this catalyst wraps metal catalysts with organic polymer materials to drive only selective chemical reactions. It is expected to maximize the efficiency of catalysts in petrochemical processes and others. On the 30th, the Korea Advanced Institute of Science and Technology (KAIST) announced that a joint research team led by Professor Minki Choi of the Department of Bio and Chemical Engineering and Professor Hyeongjun Kim of the Department of Chemistry developed a high-performance industrial catalyst, and their research results were published in Science Advances.
Development of Industrial Catalyst Resembling Enzymes
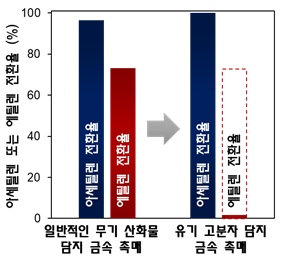 Comparison of Acetylene and Ethylene Hydrogenation Conversion Rates between Metal Catalysts Supported on Conventional Metal Oxides (Silica) and Metal Catalysts Supported on Organic Polymers
Comparison of Acetylene and Ethylene Hydrogenation Conversion Rates between Metal Catalysts Supported on Conventional Metal Oxides (Silica) and Metal Catalysts Supported on Organic Polymers
The research team developed a high-performance industrial catalyst that can selectively convert reactants based on the same principles as natural enzymes when producing chemical raw materials commonly used in daily life, such as plastics and vinyl.
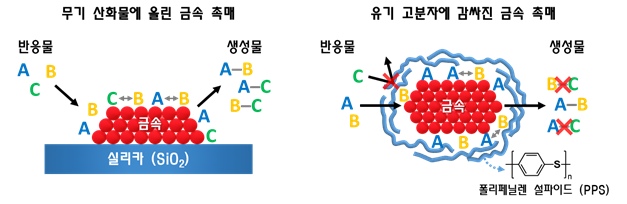
Enzymes are the most efficient catalysts existing on Earth. They are composed of proteins, which are soft organic polymers, and depending on the structure of the protein, they induce the desired chemical reactions.
The research team developed a catalyst utilizing these characteristics of enzymes. It is a catalyst in which a metal catalyst is wrapped with a polymer membrane made of polyphenylene sulfide (PPS), a plastic material that is soft and fluid like enzymes. This material has excellent heat resistance and chemical resistance and is widely used in industries such as automotive and aerospace.
Selective Reaction Only for Acetylene... Selectivity Doubled, Stability Increased Tenfold
The research team applied the developed catalyst to the acetylene hydrogenation reaction, which is the most important process in ethylene production. More than 90% of the raw materials for South Korea's petrochemical industry are naphtha, which is cracked in naphtha cracking centers (NCC) to produce ethylene and other basic petrochemical feedstocks. Ethylene, in particular, is a fundamental core chemical raw material used to make various everyday products such as common plastics, vinyl, adhesives, and paints. However, when producing ethylene by cracking naphtha, less than 1% acetylene is generated. Acetylene is a harmful substance in the production of chemical products from ethylene and must be removed.
The research team applied the newly developed catalyst to this process and selectively extracted only acetylene. Less than 1% acetylene permeated the PPS polymer membrane and underwent chemical reactions, while more than 99% ethylene was blocked by the polymer membrane, preventing catalytic reactions. The research team stated that compared to existing catalysts, the selectivity was improved more than twofold, and stability was enhanced more than tenfold.
Professor Minki Choi said, "The catalyst design method that mimics natural enzymes to selectively convert only the desired reactants while having excellent stability is a new concept that has not been reported worldwide," and added, "It is expected to be widely applied and utilized in various chemical reactions that require high selectivity in the future."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.