Elevated Status as a Model Quarantine Benchmark
Export Share Doubled Compared to Previous Year
[Asia Economy Reporter Choi Dae-yeol] Domestic pharmaceutical and bio companies are drawing attention as they continue to invest in facilities regardless of the COVID-19 pandemic. This move is in preparation for an increase in export volumes, as preference for 'K-Bio' has risen due to South Korea's successful quarantine measures. The industry expects biohealth exports to continue rising for the time being amid the global spread of COVID-19.
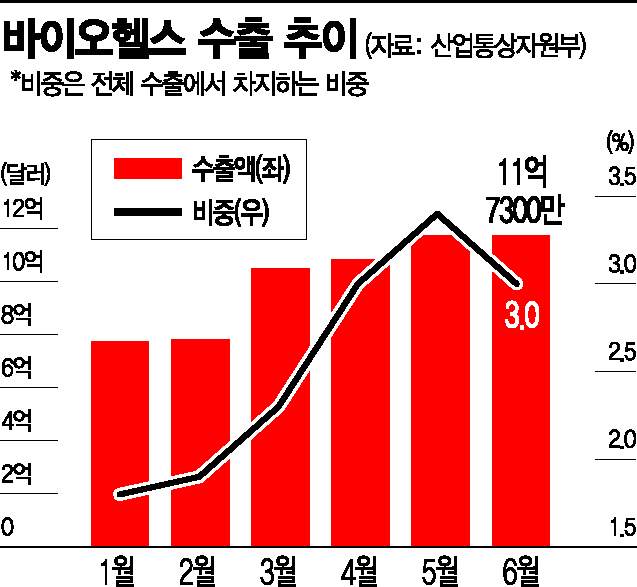
According to monthly export-import data compiled by the Ministry of Trade, Industry and Energy on the 29th, biohealth exports in the first half of this year amounted to $6.025 billion, an increase of more than 40% compared to the first half of last year. Biohealth encompasses pharmaceuticals and medical devices, and its share of South Korea's total exports was 1.6% on an annual basis last year but has recently risen to the 3% range. The growth trend has accelerated recently, with monthly exports surpassing $1 billion for the first time in March this year.
Domestic pharmaceutical and bio companies are taking proactive measures such as expanding production facilities or increasing research and development (R&D) investments, anticipating further growth in demand for Korean products on the international stage. CMG Pharmaceutical has decided to invest 100 billion KRW by 2023 to expand its factory size to meet the U.S. Food and Drug Administration (FDA) current Good Manufacturing Practice (cGMP) standards. The plan includes building new factories and research centers in Pangyo, Gyeonggi Province, to produce oral dissolving film (ODF) products and eye drops.
The company is promoting the sale of Defijoe, considered the world's first ODF schizophrenia treatment, in the U.S. using ODF technology. It applied for marketing approval in the U.S. at the end of last year and expects local sales to begin as early as the second half of this year.
A company official said, "We are also considering building a new factory dedicated to producing solid forms such as capsules, and specific plans such as site confirmation are expected to become visible within this year." He added, "Once the new factory is established, ODF production is expected to increase tenfold, and solid forms by about three times."
Ilyeon Pharmaceutical, which specializes in contrast agents, is constructing a pharmaceutical production plant in Chungju, Chungbuk, to produce biopharmaceuticals and chemically synthesized drugs with an investment of 240 billion KRW. The investment is scheduled to continue until early 2022. Raw material pharmaceutical company Chong Kun Dang Bio plans to invest 45.7 billion KRW by December next year to build a factory in Osong, Chungbuk. It is reported that they will increase probiotic production capacity, which has grown in recent years, and also equip some new drug facilities.
Large companies have also taken action. Samsung Biologics, after equipping freeze dryers and a dedicated line for small-scale clinical products, is internally reviewing plans to advance the schedule for its new fourth plant. The company mainly undertakes contract manufacturing of biopharmaceuticals, and the recent increase in orders and expansion into contract development have driven this move. Dong-A Socio Holdings Group is building a research center in Songdo, Incheon, to oversee biopharmaceutical development and a factory responsible for Dong-A ST's pharmaceutical production.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.