After US International Trade Commission Preliminary Ruling, Conflicting Claims Continue
Debate Over Evidence of Strain Misappropriation, Protection of US Companies, and Disclosure of Ruling
[Asia Economy Reporter Cho Hyun-ui] The dispute between Medytox and Daewoong Pharmaceutical over 'strain theft' has intensified following the preliminary ruling by the U.S. International Trade Commission (ITC). Although the preliminary ruling favored Medytox, both parties continue to assert differing claims regarding the core issue of strain theft, fueling a heated 'off-stage war of words.' While Medytox is expected to have the upper hand in the final ruling scheduled for November, Daewoong Pharmaceutical has expressed its intention to file a lawsuit with the U.S. Supreme Court, which serves as the appellate court for the final ruling, making a prolonged battle inevitable. Some are concerned that the exhausting conflict between the two companies could negatively impact the domestic botulinum toxin industry.
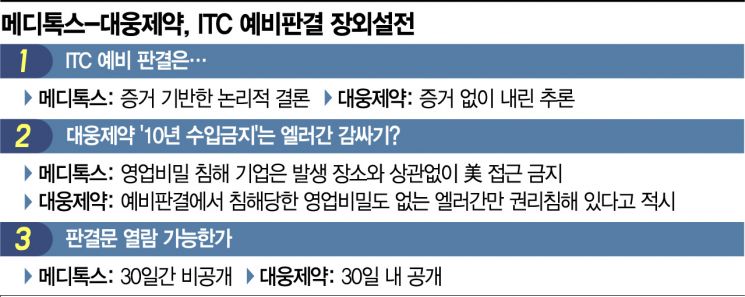
◆ Evidence-Based Conclusion or Inference= According to industry sources on the 16th, the main points of contention between Medytox and Daewoong Pharmaceutical regarding the ITC preliminary ruling are: ▲whether a decision on trade secret infringement can be made without evidence ▲whether the ruling was intended to protect the U.S. company Allergan ▲and whether the ITC preliminary ruling document should be disclosed. Daewoong Pharmaceutical argues that although the ITC administrative law judge acknowledged the failure to prove the theft of the botulinum strain, the ruling still favored Medytox. Daewoong Pharmaceutical stated, "The ITC accepted Medytox's one-sided claim that the genetic similarity between the two manufacturers' strains is relatively high and that the claim of strain collection from soil lacks credibility," adding, "Judging trade secret misappropriation based solely on inference without concrete evidence is a clear error."
In response, Medytox countered, "The ITC concluded that the DNA analysis results are definitive evidence of theft allegations," and "Daewoong Pharmaceutical attempted to undermine the credibility of the DNA analysis, but the ITC rejected the analysis results from Daewoong Pharmaceutical's experts." Medytox's strain 'HallA Hyper,' which developed Korea's first botulinum toxin preparation, does not form spores, so if Daewoong Pharmaceutical's strain forms spores, it could dispel the theft suspicion.
◆ Effectively Protecting Domestic Companies?= The second point of contention is whether the ITC issued this ruling to protect the U.S. company Allergan. Allergan, which was the first in the world to commercialize botulinum toxin, holds over 70% of the U.S. market and is Medytox's partner in the U.S. market. Daewoong Pharmaceutical's botulinum toxin preparation 'Nabota' entered the U.S. market last year as the first domestically produced botox product. However, due to the ITC's recent '10-year import ban' ruling, Daewoong Pharmaceutical now faces the prospect of abandoning its ambition to become the second-largest player in the U.S. botox market.
Daewoong Pharmaceutical is protesting, claiming that the U.S. ITC ruling sided with the domestic company Allergan. Daewoong Pharmaceutical stated, "The ITC protected only Allergan, a U.S. company with no trade secrets infringed," calling it "an unprecedented case beyond ITC jurisdiction." On the other hand, Medytox rebutted, "The ITC has prohibited access to the U.S. market for products resulting from trade secret theft regardless of where the illegal act occurred." Medytox dismissed claims that the ITC sided with domestic companies as "a frame unrelated to the essence." Medytox exported its liquid botox 'Innotox' to Allergan in 2013 for $360 million (approximately 400 billion KRW) in technology transfer fees but has yet to enter the U.S. market.
◆ '30 Days Confidential' VS 'Disclosure Within 30 Days' = On the 13th, Daewoong Pharmaceutical announced that after analyzing the ITC's decision document, it confirmed a significant error by the ITC and claimed to have directly reviewed the preliminary ruling document. In response, Medytox stated on the 14th, "The ITC's preliminary ruling document is designated as 'confidential' for 30 days," and accused Daewoong Pharmaceutical of making false claims or violating regulations by viewing the ruling document without authorization. The ITC posted on its website on the 7th that the 'public version' of the final preliminary ruling is scheduled to be disclosed within 30 days. This means it will be disclosed within 30 days, not kept confidential for 30 days. A Daewoong Pharmaceutical official said, "Our company received a summary of the ruling unrelated to trade secrets from a local U.S. law firm," adding, "According to U.S. attorney regulations, unauthorized disclosure of confidential rulings can lead to disbarment. There is no reason for the U.S. law firm to risk such consequences by disclosing the ruling to us."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.