Development of the World's Second Standard Material
Containing 90% of COVID-19 Genome Information
Measuring the Number of COVID-19 Genes in Samples
Can Be Used to Assess Diagnostic Kit Accuracy
 This is a standard genetic material for the COVID-19 virus developed by the Korea Research Institute of Standards and Science. Inside the small vial is the standard material used in diagnostic kits to determine the presence of the COVID-19 virus. Utilizing this material improves the accuracy of infection diagnosis in the diagnostic kits.
This is a standard genetic material for the COVID-19 virus developed by the Korea Research Institute of Standards and Science. Inside the small vial is the standard material used in diagnostic kits to determine the presence of the COVID-19 virus. Utilizing this material improves the accuracy of infection diagnosis in the diagnostic kits.
[Asia Economy Reporter Hwang Junho] South Korea has developed the world's second standard material for the novel coronavirus disease (COVID-19) gene, following China. This material contains information covering 90% of the COVID-19 genome and is expected to be used to improve the accuracy of diagnostic kits and respond to virus mutations.
The Korea Research Institute of Standards and Science (KRISS) and the Novel Virus Research Group announced on the 14th that they have developed the first domestic standard material for the COVID-19 virus gene.
Development of Korean-made COVID-19 Standard Material
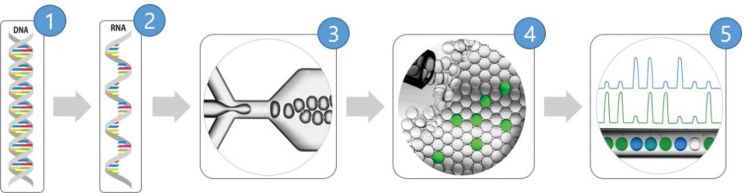
The standard material developed by the research team serves as a reference material to evaluate the accuracy of diagnostic kits. The team developed the standard material domestically for the first time using 'Reverse Transcription Digital Polymerase Chain Reaction (RT-dPCR)'.
RT-dPCR is a type of polymerase chain reaction technology that amplifies target genes by creating microdroplets or partitioning the reaction mixture into small compartments. This method reads fluorescent signals emitted during the amplification of specific gene sequences after reverse transcription of RNA into DNA, offering higher accuracy than the conventional 'Reverse Transcription Polymerase Chain Reaction (RT-PCR)'.
The research team stated that their standard material is more accurate than the only existing Chinese standard material. The Korean standard material utilizes the RT-dPCR method, enabling absolute quantification of genes within samples. This is possible because the number of genes can be measured based on the count of microdroplets or partitions. In particular, the newly developed standard material contains RNA covering 90% of the COVID-19 genome information, allowing it to respond to virus mutations. In contrast, the Chinese material contains only 10% of the COVID-19 genome information.
Immediately Usable for Measuring Diagnostic Kit Accuracy
The research team explained that the standard material can help detect errors in diagnostic kits. By using the gene count of the standard material as a reference, it is possible to reduce errors such as false negatives (cases where infection is present but diagnosed as negative) and false positives (cases where infection is absent but diagnosed as positive).
Senior Researcher Kim Se-il stated, "We are also developing standard materials in the form of virus particles that more closely resemble COVID-19." Senior Researcher Bae Young-kyung added, "The newly developed standard material will assist in quality control of domestically produced diagnostic kits, which have been actively exported recently." The developed standard material can be immediately used to assess the accuracy of domestic diagnostic kits.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.