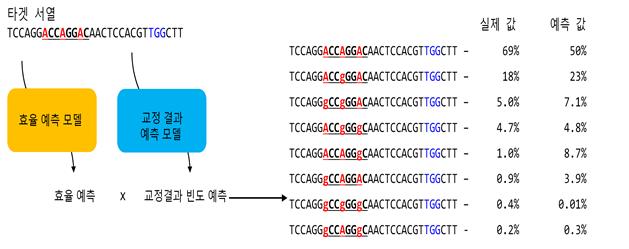
 This is a schematic diagram of the prediction program. When the nucleotide sequence to be corrected is input, two prediction models respectively quantify the base correction efficiency and the frequency of possible correction outcomes. Then, the two models are combined to perform predictions on all possible outcomes that the base editing gene scissors can produce.
This is a schematic diagram of the prediction program. When the nucleotide sequence to be corrected is input, two prediction models respectively quantify the base correction efficiency and the frequency of possible correction outcomes. Then, the two models are combined to perform predictions on all possible outcomes that the base editing gene scissors can produce.
[Asia Economy Reporter Junho Hwang] An artificial intelligence (AI) that can maximize the effectiveness of base-editing gene scissors, which correct genes by cutting out bases like scissors, has been developed by a domestic research team. This AI uses deep learning on efficiency and outcome data by base position to infer diseases that can be treated with base-editing gene scissors or predict various base-editing outcomes.
Professor Hyungbeom Kim's research team at the National Research Foundation of Korea announced on the 8th that they developed an AI program capable of measuring the efficiency of base-editing gene scissors and predicting correction results. The team's research was published in Nature Biotechnology, an international journal in the field of biotechnology.
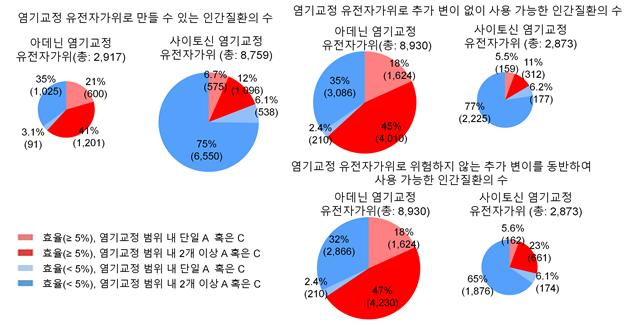 This table shows the predicted efficiency of base-editing gene scissors for human point mutation diseases. The prediction program suggests the applicability for diseases marked in dark red (efficiency above 5%, with two or more A or C bases within the base-editing range), while it predicts difficulty in use for diseases marked in light blue (efficiency below 5%, with a single A or C base within the base-editing range). This allows for an initial screening of target diseases for base-editing gene scissors.
This table shows the predicted efficiency of base-editing gene scissors for human point mutation diseases. The prediction program suggests the applicability for diseases marked in dark red (efficiency above 5%, with two or more A or C bases within the base-editing range), while it predicts difficulty in use for diseases marked in light blue (efficiency below 5%, with a single A or C base within the base-editing range). This allows for an initial screening of target diseases for base-editing gene scissors.
The research team designed the program to enhance the therapeutic effect of base-editing gene scissors on "point mutations," which are the cause of genetic diseases and gene mutations. Base-editing gene scissors can correct point mutations by changing specific bases. However, if multiple identical bases exist within a certain base sequence, there is a possibility of editing unintended bases. Therefore, it is important to predict editing frequency by position and use safe gene scissors.
The team created various base-editing gene scissors and secured big data on the efficiency and frequency of each outcome, analyzing it with deep learning to develop an AI program that predicts base-editing results.
This AI identified 3,058 diseases with high therapeutic efficiency of gene scissors among 23,479 point mutation diseases. These diseases are expected to show over 5% efficiency with a single target base, such as cystic fibrosis.
The team also found 4,274 diseases predicted to have over 5% efficiency and no mutations in other bases among 19,505 diseases where two or more identical bases (adenine or cytosine) exist within the same range, which could lead to editing at unintended positions. These selected genetic diseases have two or more target bases and were previously considered difficult to treat with gene scissors due to the possibility of additional mutations.
 Research Fellow Hyungbeom Kim, IBS Center for Nanomedicine
Research Fellow Hyungbeom Kim, IBS Center for Nanomedicine
The research team stated, "The activity prediction model showed a high reliability with a correlation coefficient converging between 0.69 and 0.79 between actual experimental results and AI-predicted values," and "The outcome prediction model showed an even higher correlation coefficient between 0.91 and 0.93." A correlation coefficient closer to 1 indicates greater accuracy and reliability. The team also verified the AI's results using human-induced pluripotent stem cells.
The team added, "This will greatly assist researchers in setting directions and strategies for studies based on the initially selected gene scissors and disease information." They plan to use this AI to apply the selected gene scissors in animal model treatments.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















