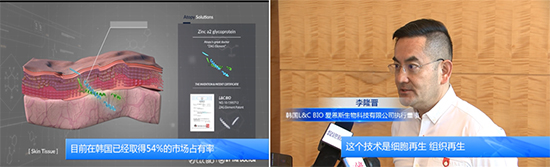
Chinese state-run media reported that L&C Bio (CEO: Lee Hwan-cheol) has established a subsidiary in China and completed the registration of the subsidiary in Kunshan City. The main interview content conveyed through Chinese state broadcasting was that “L&C Bio, a bio company based on regenerative medical engineering technology, holds over 50% market share in the human tissue implant market in Korea, and has officially entered the Chinese market through the establishment and registration of a subsidiary in China.”
L&C Bio established a subsidiary in Kunshan, China, last June and is advancing into the Chinese human tissue implant market, which has a market size of over 2 to 3 trillion KRW, through the establishment of a joint venture in cooperation with China International Capital Corporation (CICC) and others. Additionally, to expedite approval processes in China, they are preparing for approvals in collaboration with top Chinese medical device experts and CRO companies.
Kunshan City held its own media event called the ‘Kunshan Major Investment Company Contract Ceremony’ to introduce L&C Bio, pharmaceutical companies, and medical device companies to Chinese and regional media outlets.
Kunshan City is a developed city with the highest per capita GDP in China at $34,798, approximately 1.5 times higher than Beijing ($21,188), ranked second, and Shanghai ($20,421), ranked third. It has been designated as a national-level development zone, including the Kunshan Medical Equipment Tech Industrial Park, where China’s five-year bio plan has been established, attracting multinational companies from over 50 countries worldwide.
Furthermore, foreign companies investing in the Kunshan Medical Park receive financial benefits of up to 50 million yuan, and key personnel can receive up to 6 million yuan, with government-level support provided to facilitate rapid approval processes.
An L&C Bio official stated, “Regarding the NMPA (formerly CFDA) medical device approval for human tissue products, although it varies by product, we plan to proceed with the approval process aiming for about one year for Class 3 products, and expect the approval process for Class 2 products to be completed within a few months.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.