[Asia Economy Reporter Cho Hyun-ui] The Ebola virus treatment drug 'Remdesivir,' which has shown effectiveness in treating severe cases of the novel coronavirus infection (COVID-19) in the United States, will be supplied in South Korea.
The Korea Disease Control and Prevention Agency (KDCA) announced that it has signed a contract for the free supply of the drug through domestic introduction negotiations with Gilead Sciences Korea and will begin supplying it from the 1st.
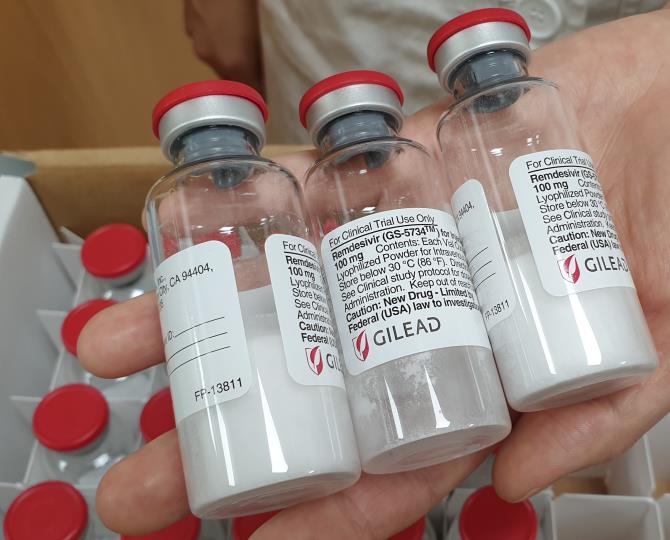
Remdesivir is a drug developed by Gilead Sciences as a treatment for COVID-19 and has been granted emergency use authorization by the U.S. Food and Drug Administration (FDA) for treating severe COVID-19 patients only. Recent early clinical trials conducted in the U.S. reported that it reduced the recovery time of COVID-19 patients by 31%, drawing attention to its therapeutic effects.
In South Korea, patients eligible to receive Remdesivir are limited to severe cases with pneumonia requiring oxygen therapy. Hospitals treating severe patients must request drug supply from the National Medical Center, which, if necessary, consults the Central Clinical Committee on Emerging Infectious Diseases to determine the recipients of the drug.
The KDCA plans to secure the free supply quantity by the end of this month and proceed with purchasing through price negotiations starting next month.
Jung Eun-kyung, head of the KDCA, stated, “We will do our best to secure the treatment drug by continuously cooperating with Gilead Sciences Korea, the domestic importer, to obtain additional quantities of Remdesivir.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














