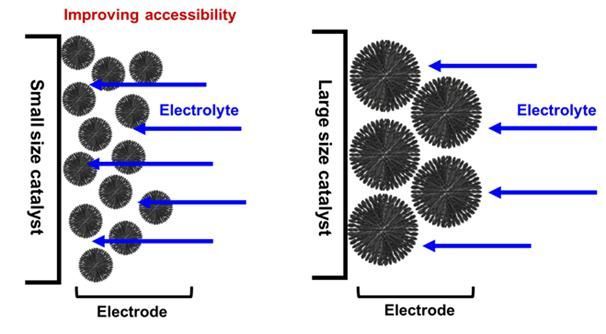
 A diagram illustrating electrolyte accessibility according to carbon particle size. Smaller particle sizes allow easier electrolyte access, resulting in superior oxygen reduction reaction performance.
A diagram illustrating electrolyte accessibility according to carbon particle size. Smaller particle sizes allow easier electrolyte access, resulting in superior oxygen reduction reaction performance.
[Asia Economy Reporter Junho Hwang] A catalyst for oxygen reduction reaction that can develop hydrogen fuel cells at a low cost has been developed. It is expected to be a technology that will contribute to the commercialization of hydrogen fuel cells in various fields such as automobiles and power plants. The research team led by Professor Chanho Park of the School of Convergence Technology at Gwangju Institute of Science and Technology (GIST) announced on the 20th that they developed a non-platinum catalyst that is cheaper than platinum and has excellent performance under alkaline conditions.
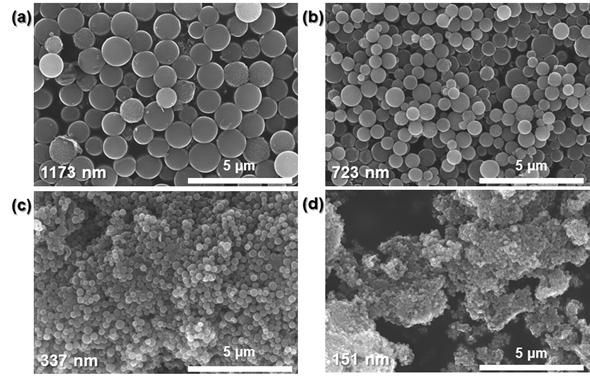 This is a scanning electron microscope image of the old catalyst. The sizes of each catalyst are (a) 1173 nm, (b) 723 nm, (c) 337 nm, and (d) 151 nm.
This is a scanning electron microscope image of the old catalyst. The sizes of each catalyst are (a) 1173 nm, (b) 723 nm, (c) 337 nm, and (d) 151 nm.
The research team developed a platinum-free catalyst using a nano-templating method by impregnating iron and nitrogen precursors (compounds) into porous spherical silica, a ball-shaped silicon oxide with numerous pores. They also confirmed that this catalyst induces superior oxygen reduction reaction compared to platinum through the bonding between iron and nitrogen. The nano-templating method is a technique to obtain porous materials by using a hard metal oxide with pores as a template. The precursor of the material to be manufactured is placed inside the pores of the template, solidified, and then the template is removed to obtain the desired material.
In particular, the research team adjusted the size of the iron-nitrogen catalyst to achieve the maximum efficiency of the catalytic reaction. They confirmed that the smaller the size of the carbon particles, the larger the contact area between the carbon particles and the electrolyte, improving accessibility and thus promoting the oxygen reduction reaction. Considering performance degradation, they also derived the most efficient particle size within the approach used in this experiment.
Professor Chanho Park said, "This research is significant in that it developed a non-platinum oxygen reduction catalyst applicable to anion exchange membrane hydrogen fuel cells," adding, "It is expected to help reduce the cost of hydrogen fuel cells through the development of inexpensive new oxygen reduction catalysts in the future, and we hope it will contribute to widespread commercialization."
The research results were published in the Journal of Energy Chemistry, an academic journal in the field of applied chemistry.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














