Botox 'Medytoxyn Injection' Manufacturing and Sales Suspension Order
"Passed MFDS Inspection... No Safety or Efficacy Issues"
Approval Competency Questioned Following Inbosa Incident
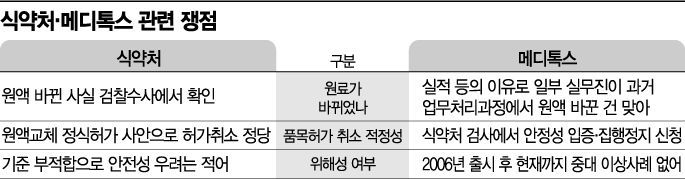
[Asia Economy Reporters Choi Dae-yeol, Cho Hyun-ui] Medytox has filed an administrative lawsuit in response to the Ministry of Food and Drug Safety's (MFDS) decision to revoke the approval of its botulinum toxin (Botox) product. The company argues that there is no risk from products manufactured in the past, and that the MFDS's repeated inspections previously deemed the products compliant, making the MFDS's actions unreasonable. As the legal battle between Medytox, the leading Botox company in the domestic market, and the MFDS intensifies, the Korean pharmaceutical and biotech industry is expected to face another controversy following the Kolon Inbosa incident.
"Products Manufactured in the Past Are No Longer Sold"
On the 20th, Medytox announced that it had filed a suspension of execution request and a cancellation lawsuit at the Daejeon District Court regarding the MFDS's administrative order. The Korea Exchange's KOSDAQ Market Headquarters suspended trading of the company's shares starting that day in accordance with relevant regulations. Earlier, on the 17th, the MFDS issued a provisional manufacturing and sales suspension order for Medytoxyn Injection (50, 100, 150 units).
Additionally, the MFDS is proceeding with the cancellation of the product's approval. Following a public interest report last year alleging falsification of test reports for Medytoxyn Injection, the MFDS requested a prosecution investigation. As a result of the investigation, the company was prosecuted on charges including manufacturing products with unauthorized raw materials and falsifying information, leading the MFDS to impose administrative sanctions.
The company maintains that the MFDS's actions lack sufficient grounds. Considering that administrative procedures take time, the MFDS issued a provisional manufacturing and sales suspension; however, the products in question were manufactured between December 2012 and June 2015 and are no longer available. The company emphasized, "The MFDS order presumes a 'public health hazard,' but the products were already exhausted long ago and no longer exist. Therefore, there can be no public health hazard at this point."
According to the company, currently distributed Medytoxyn Injection products have been manufactured since April 2017. Previous inspections conducted by the MFDS in 2016 and 2018 on distributed products resulted in compliance. Furthermore, special pharmacist surveillance and random sampling inspections conducted last year confirmed that products within their expiration dates showed no issues regarding safety or efficacy, the company explained.
MFDS Approval Competency Questioned Following Kolon Inbosa Incident
MFDS Acknowledges Pharmaceutical Management Issues
Amid ongoing controversy surrounding Kolon Life Science's osteoarthritis treatment 'Inbosa K Injection,' the dispute over Medytoxyn has further raised questions about the MFDS's approval capabilities. In the case of Inbosa, the approval was revoked after it was confirmed that the product contained different ingredients than those originally submitted.
Considering that Medytoxyn Injection was the first domestically developed Botox product in 2006 and has been produced for over a decade, widely used both domestically and internationally, the MFDS is unlikely to escape responsibility for oversight. According to the company, the suspended product accounts for approximately 86.8 billion KRW in annual domestic and international sales, representing over 40% of annual revenue. Industry insiders estimate that Medytox holds nearly half of the domestic Botox market share.
The MFDS itself acknowledges issues in the pharmaceutical management system. While proceeding with the cancellation of Medytoxyn's approval, the MFDS stated it will "introduce a system to track and verify data modification histories for items with a high possibility of data manipulation during pharmaceutical manufacturing and quality control."
For low-risk pharmaceuticals, testing was previously exempted, and only submitted documents were reviewed; however, the MFDS now plans to conduct random testing. This implies that until now, there was a lack of mechanisms to prevent data falsification by companies. Industry experts point out that the MFDS has faced a persistent shortage of evaluation and review personnel for several years, and that structural problems have emerged due to conflicts of interest arising from simultaneously handling regulatory approval tasks and industry promotion roles.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














