
[Asia Economy Reporter Junho Hwang] Domestic researchers have developed a technology that induces hydrogenation reactions?used in the food and petrochemical industries?at room temperature and low cost. Since it does not use expensive precious metal catalysts, cost reductions are expected across related industries. The joint research team led by Youngkyu Hwang, Director of the Chemical Process Research Division at the Korea Research Institute of Chemical Technology, and Professor Jaehun Jeong of Ulsan University published their findings on this hydrogenation reaction catalyst as a cover paper in the March issue of the world-renowned catalysis journal, ACS Catalysis.
Affordable Hydrogenation Reactions at Room Temperature
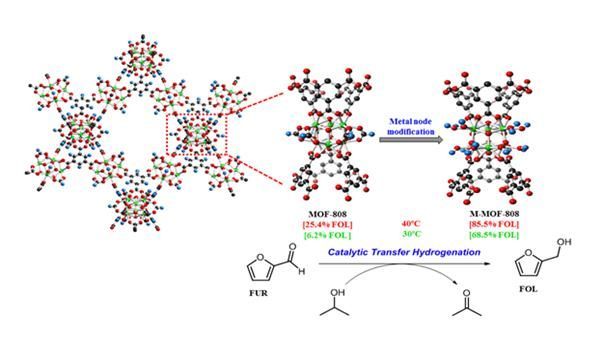 This illustration shows the conversion of furfural, a biomass, into furfuryl alcohol, a chemical raw material, at room temperature through the reaction of a newly developed metal-organic framework (MOF) catalyst. The research team enhanced the stability of the MOF catalyst by activating its surface with alcohol. They also expanded the reaction area. As a result, the activation energy of the hydrogenation reaction was lowered, enabling easy production of chemical raw materials from biomass even at room temperature below 30°C.
This illustration shows the conversion of furfural, a biomass, into furfuryl alcohol, a chemical raw material, at room temperature through the reaction of a newly developed metal-organic framework (MOF) catalyst. The research team enhanced the stability of the MOF catalyst by activating its surface with alcohol. They also expanded the reaction area. As a result, the activation energy of the hydrogenation reaction was lowered, enabling easy production of chemical raw materials from biomass even at room temperature below 30°C.
The research team succeeded in inducing hydrogenation reactions at room temperature by simply heating alcohol with a self-developed metal-organic framework (MOF) catalyst. They found that when alcohol is boiled with the MOF catalyst, hydrogenation reactions occur at the zirconium oxide sites of the MOF. Furthermore, the number of reaction sites gradually increased, and even when the temperature was lowered to 30°C at room temperature, hydrogenation reactions occurred easily.
The researchers also converted biomass-derived furan compound 'Furfural' into the chemical raw material 'Furfuryl alcohol' at room temperature using the MOF catalyst. In addition, they produced seven types of intermediates used in petrochemical and biochemical processes through this method.
Hydrogenation is a chemical reaction in which a liquid hydrogen source and reactants are introduced together with a catalyst to obtain new products. It is widely used to synthesize intermediates in petrochemical processes producing plastics, fuels, fibers, and rubber; intermediates in fine chemical processes producing pharmaceuticals and cosmetics; and biomass in biochemical processes. Generally, hydrogenation reactions occur at high temperatures above 100°C. When hydrogenation is induced at room temperature, expensive catalysts such as palladium and platinum must be used.
Hydrogenation Reaction Using Waste Heat
 Photo by Getty Images
Photo by Getty Images
The research team expects that applying their self-developed catalyst to existing processes could induce hydrogenation reactions using waste heat generated from those processes. Waste heat generated in petrochemical processes is typically above 80°C. Usually, petrochemical plants cool this waste heat with cooling water to below 40°C before releasing it into the air to minimize environmental impact. The research team anticipates that utilizing this cooling water-cooled waste heat as the reaction heat for the new hydrogenation reaction will not only recycle waste heat but also contribute to reducing greenhouse gas emissions.
Additionally, the team newly elucidated the hydrogen transfer mechanism in the hydrogenation reaction. Through actual experiments and computational science, they revealed that the hydrogenation reaction proceeds via an eight-membered ring transition state rather than the six-membered ring transition state involving the catalyst and reactants. For the past decade, researchers have developed catalysts based on the six-membered ring transition state explained by Professor James A. Dumesic of the Department of Chemical and Biological Engineering at the University of Wisconsin. It turns out the transition state was misunderstood until now.
Youngkyu Hwang, Director of the Korea Research Institute of Chemical Technology, stated, "Since the intermediate transition state of the hydrogen transfer process has been identified, active research on new catalytic reaction pathways based on this will be conducted," adding, "This will have a significant impact on petrochemical, fine chemical, and biochemical processes."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















