'Passage of the Data 3 Act: From Opening to Utilizing Medical Data' Report Published
[Asia Economy Reporter Minji Lee] With the passage of the Data 3 Act this year expected to mark a turning point for the digital healthcare industry, there are calls to continuously discover large-scale government-led projects to lower the barriers for companies to enter the healthcare industry.
According to the report titled “Passage of the Data 3 Act: From Opening to Utilizing Medical Data” published by Samjong KPMG on the 30th, South Korea is evaluated as an environment conducive to the growth of digital healthcare companies, but the technological capabilities of digital healthcare companies in the global market have not stood out.
Among the top 100 global digital healthcare startups by cumulative investment since 2014, the United States accounted for 72 companies, followed by the United Kingdom (4), India (4), Sweden (3), and France (3).
The report explained that despite South Korea’s globally high level of medical data holdings and infrastructure penetration, no domestic companies have made it into the top 100 global digital healthcare startups by cumulative investment.
The overall average technological capability of Korean healthcare is about 4.5 years behind that of the United States. The number of patent applications related to personalized healthcare filed with the top five global patent offices (United States, Europe, China, Japan, South Korea) was about 7% (1,588 cases) of the U.S. level, recording the lowest number among the surveyed countries. Additionally, 63 of the top 100 global digital healthcare startups reported that their business operations in South Korea are restricted due to domestic regulations.
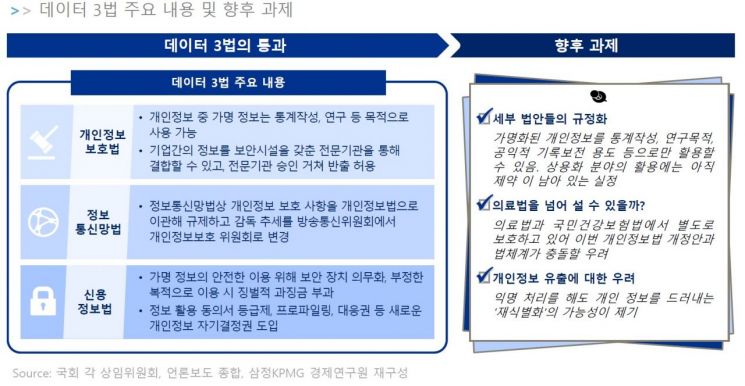
Accordingly, the report suggested that for the effective operation of the Data 3 Act, large-scale projects should be continuously discovered to lower the entry barriers to the healthcare industry. It also explained that policy changes are needed to improve entry regulations, such as legislating the concept of de-identified medical information, clarifying regulations on autonomous utilization, gradually expanding the scope of telemedicine, and expanding the allowable items for DTC genetic testing.
Notable global cases introduced include ▲the U.S. ‘All-of-US’ program, which enabled tech companies with digital capabilities to enter the healthcare market based on a large-scale government cohort construction ▲Japan’s ‘Next-Generation Medical Infrastructure Act,’ which classified health information as ‘necessary consideration personal information’ and actively increased data utilization ▲Finland’s ‘Biobank’ and ‘Kanta system,’ which centralized all large-scale healthcare-related data.
The report emphasized that companies must also prepare technical countermeasures for utilizing medical big data. Advanced overseas companies are strengthening their own capabilities in line with active government support policies for medical big data by ▲training specialists in big data management and analysis ▲investing in research, development, and learning of software used for big data analysis ▲advancing cloud technology to secure storage space for medical big data.
Kyungsoo Park, Healthcare Industry Leader at Samjong KPMG, said, “For the growth of digital healthcare, it is important to connect one person’s genetics, medical records, and life log,” adding, “The government should lower the barriers for companies to enter the healthcare industry, and companies should enhance competitiveness through acquisitions or collaborations with ICT companies.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.