[Asia Economy Reporter Eunmo Koo] Cancerop, a molecular diagnostic company specializing in genetic analysis, announced on the 12th that it has obtained European certification (CE-IVD) and export approval from the Ministry of Food and Drug Safety for its novel coronavirus disease (COVID-19) diagnostic kit, ‘Q-Sens 2019-nCoV Detection Kit.’
The Ministry of Food and Drug Safety, through the ‘Submission Materials for Export COVID-19 Diagnostic Reagent Approval Application’ announced on the 19th of last month, requires submission of clinical evaluation data for export approval of this COVID-19 diagnostic kit, unlike the existing export approval for diagnostic kits. This implements export approval for COVID-19 diagnostic reagents with strengthened requirements compared to the domestic emergency use authorization system, which only evaluates performance data from diagnostic reagent manufacturers.
A Cancerop official explained, “The strengthened export approval requirements for the COVID-19 diagnostic kit appear to consider the international reliability of domestically produced diagnostic kits. Cancerop’s diagnostic reagents underwent clinical evaluation to prove safety and efficacy at Myongji Hospital, a COVID-19 base hospital, among other efforts, achieving excellent results. The Ministry of Food and Drug Safety recognized the validity of these clinical results, enabling us to obtain approval.” The official added, “We produce main raw materials such as diagnostic reagents in-house at Cancerop’s KGMP (Korea Good Manufacturing Practice) production facility, and we are currently in rapid discussions for raw material exports as well as the diagnostic kits.”
Cancerop completed the development of the diagnostic kit at the end of January and applied for emergency use authorization for infectious disease in vitro diagnostic products from the Korea Disease Control and Prevention Agency. After obtaining European CE certification, the company is currently negotiating export contracts with partners in China, Southeast Asia, South Africa, and Europe for rapid commercialization.
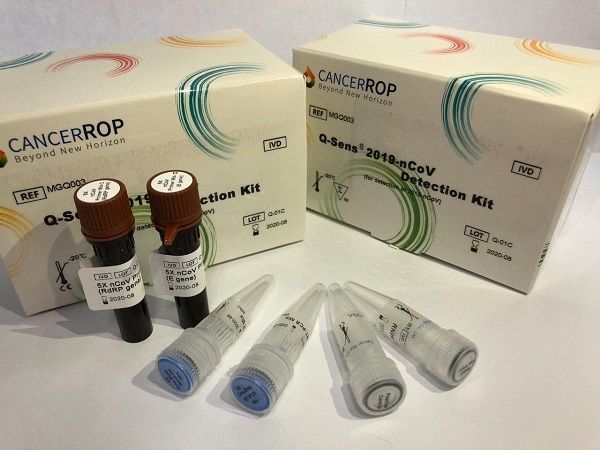
The ‘Q-Sens 2019-nCoV Detection Kit’ developed by Cancerop increases diagnostic accuracy to 99.9% and reduces testing time to within 2 hours. It tests RNA (ribonucleic acid) extracted from samples such as sputum, nasopharyngeal swabs, and bronchoalveolar lavage fluid of suspected infected individuals using real-time reverse transcription polymerase chain reaction (RT-PCR).
A company representative stated, “We will promptly respond to domestic demand as soon as the Korea Disease Control and Prevention Agency completes its emergency use authorization review process, simultaneously with overseas exports,” adding, “We are proceeding swiftly to prevent the spread of infection.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















