[Asia Economy Reporter Junho Hwang] A new catalyst has been developed that produces synthesis gas by mixing major greenhouse gases carbon dioxide and methane. This is expected to achieve a twofold effect by significantly reducing greenhouse gases while industrially producing useful synthesis gas.
KAIST announced on the 17th that a research team led by Jafar Yabuz from the Department of Bio and Chemical Engineering developed a catalyst that induces the dry reforming reaction of methane without coking or sintering even after long-term use.
This research is significant because it developed a catalyst that stably induces the catalytic reaction of carbon dioxide and methane. Technologies that reduce greenhouse gases, the main cause of global warming, while producing industrially useful synthesis gas by utilizing the catalytic reaction of carbon dioxide and methane have already been developed. However, industrial application was impossible due to coking, where carbon atoms adhere to the catalyst and reduce its function, and sintering, where particles agglomerate.
Blocking the edges of the catalyst
The research team proposed nickel-molybdenum alloy nanoparticles as a solution. Observing that coking occurs at the edges of magnesium oxide during the catalytic reaction of carbon dioxide with magnesium oxide, they supported nickel-molybdenum alloy nanoparticles on single-crystal magnesium oxide supports.
As a result, when sufficient thermal energy was supplied, it was confirmed that nickel-molybdenum nanoparticles migrated over the surface of single-crystal magnesium oxide and covered thermodynamically unstable corners, enabling stable catalytic activity.
Additionally, to apply the developed catalyst to the temperature-sensitive dry reforming reaction of methane, the team measured activity while varying the temperature. They confirmed that catalytic activity remained stable even in the 700?800°C range. Even when the temperature was lowered to room temperature during the reaction and then restarted, the activity was maintained.
In particular, to apply the catalyst under industrial reaction conditions, the team applied a pressure of 15 bar, and the catalytic reaction remained stable. They also reported that no coking or sintering occurred even after 850 hours of use at 800°C.
Commercialization of 'Carbon dioxide + Methane = Synthesis Gas'
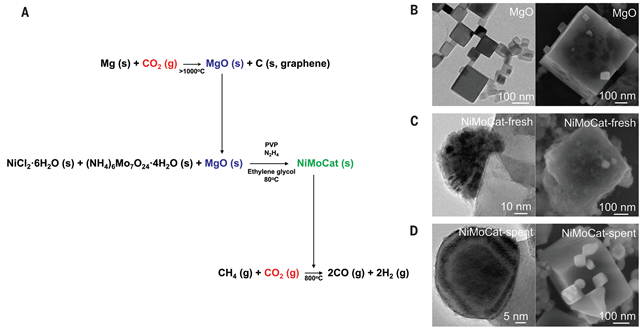 Synthesis method of single-crystal magnesium oxide and catalyst. (B) Electron microscopy image of single-crystal magnesium oxide. (C) Electron microscopy image of the synthesized catalyst. (D) Electron microscopy image of the catalyst after the dry reforming reaction of methane. Nickel-molybdenum nanoparticles are located at the vertex of the single-crystal magnesium oxide.
Synthesis method of single-crystal magnesium oxide and catalyst. (B) Electron microscopy image of single-crystal magnesium oxide. (C) Electron microscopy image of the synthesized catalyst. (D) Electron microscopy image of the catalyst after the dry reforming reaction of methane. Nickel-molybdenum nanoparticles are located at the vertex of the single-crystal magnesium oxide.
The research team stated that this catalyst can also be used for the wet reforming reaction of methane, along with the dry reforming reaction. The wet reforming reaction of methane currently accounts for more than 90% of hydrogen production methods. Through this, the team expects reductions in synthesis gas production costs, production of inexpensive nickel-based catalysts, and performance enhancement.
Song Youngdong, a doctoral candidate and first author of this study, said, "We developed a catalyst that can solve the long-standing problem of coking without expensive precious metals or complicated manufacturing processes," adding, "If the technology to stabilize nanoparticles on single crystals is applied using other supports and metal nanoparticles, various problems can be solved."
This research was conducted with support from the Saudi Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.