Identifying the Cause of Capacity Degradation in High-Output Iron-Chromium Flow Batteries
Maintaining Performance by Adjusting Electrolyte Composition... Published in Angewandte Chemie International Edition
A new technology has been developed that can extend the lifespan of the "iron-chromium flow battery," a large-capacity energy storage system (ESS) that does not pose an explosion risk.
This opens up a way to safely store electricity generated from renewable energy sources such as wind and solar power, which have inconsistent power output, and use it when needed.
The research team led by Professor Lee Hyunwook from the Department of Energy and Chemical Engineering at UNIST, in collaboration with Professor Seo Donghwa from KAIST and Professor Guihua Yu from the University of Texas, has identified the cause of performance degradation in iron-chromium flow batteries. They developed a battery that maintains its capacity even after repeated charging and discharging cycles by adjusting the composition of the electrolyte.
 Research team, (from left) Professor Hyunwook Lee, Researcher Vidya Muralidaran (first author), Dr. Jieun Jang (first author) Provided by UNIST
Research team, (from left) Professor Hyunwook Lee, Researcher Vidya Muralidaran (first author), Dr. Jieun Jang (first author) Provided by UNIST
Unlike conventional batteries, flow batteries use electrode materials dissolved in water as an electrolyte. The electrolyte itself acts as a "liquid electrode." To store or discharge electricity, the electrolyte simply needs to be pumped through the system. Since water is used instead of a volatile electrolyte, there is no risk of explosion. The storage capacity can be freely adjusted by controlling the amount of electrolyte in the tank, making it suitable for storing renewable energy sources such as wind and solar power, where power generation is unpredictable.
The joint research team identified the cause of performance degradation in high-output iron-chromium flow batteries and designed an improved electrolyte. Iron-chromium flow batteries are highly cost-competitive, but due to the low reactivity of chromium, they have slow charging speeds and low output. Adding a substance called hexacyanochromate ([Cr(CN)6]4-/3-) can improve output and charging speed, but the capacity drops sharply as charging and discharging cycles progress.
According to the research team, this is due to the replacement of cyanide (CN-) ions surrounding the chromium ions with hydroxide (OH-) ions. During the charging process, an excess of hydroxide ions is generated, and these ions occupy the positions of the cyanide ions, disrupting the structure of the electrolyte.
The team derived an optimal electrolyte composition ratio that suppresses this reaction and maintains the chemical structure of the electrolyte by controlling the concentration ratio of cyanide ions to hydroxide ions in the electrolyte. Iron-chromium flow batteries using this ratio maintained their capacity and efficiency even after more than 250 charge-discharge cycles.
Professor Lee Hyunwook explained, "This study demonstrates the possibility of producing long-lasting, high-output flow batteries using inexpensive iron-chromium electrolytes. Countries such as China and European nations, which have high renewable energy generation and enough land area to install flow batteries, are showing great interest in this technology."
Among flow batteries, vanadium flow batteries are the closest to commercialization. However, vanadium is expensive and is a mineral resource concentrated in specific regions.
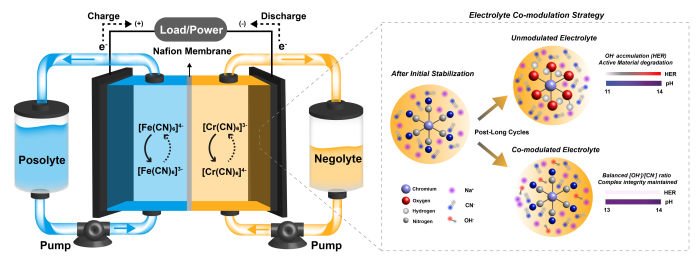 Schematic diagram of the operating principle of the iron-chromium flow battery and electrolyte stabilization strategy.
Schematic diagram of the operating principle of the iron-chromium flow battery and electrolyte stabilization strategy.
This research was supported by the International Cooperation Development Project for Fundamental Technology, the Individual Research Project, and the Global TOP Strategic Research Group Project of the National Research Council of Science and Technology, all funded by the National Research Foundation of Korea under the Ministry of Science and ICT.
The research results were published online on July 2 in Angewandte Chemie International Edition, a leading academic journal in the field of chemistry.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.











