Completion of Technology Transfer and Production Transition
for Three Major LBA Products Including Gemzar and Zyprexa
On July 9, Boryung announced that it has completed the transition to in-house production of the non-small cell lung cancer treatment 'Alimta' (active ingredient: pemetrexed). Alimta is an original pharmaceutical product that Boryung acquired from the global pharmaceutical company Eli Lilly in 2022, and now, all manufacturing processes will be carried out domestically.
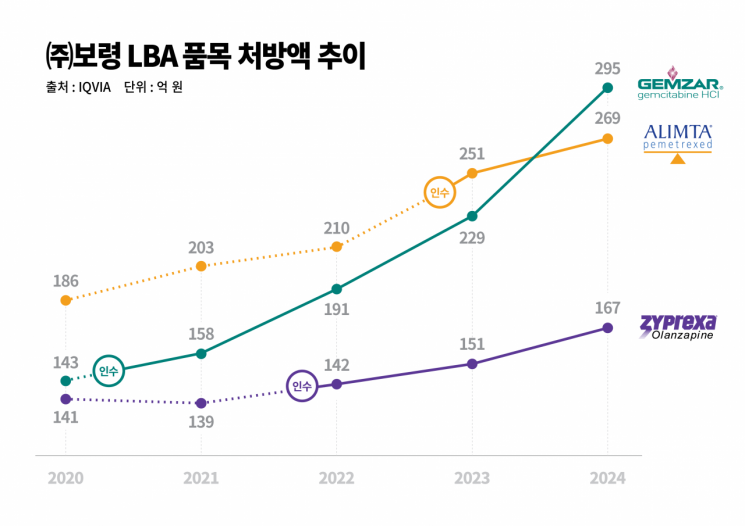
Boryung is pursuing the 'LBA (Legacy Brands Acquisition)' strategy, which involves acquiring and internalizing the production of pharmaceuticals that can maintain a certain level of sales volume and market share due to high brand loyalty even after patent expiration, and then supplying these products. Through this strategy, Boryung has sequentially acquired domestic rights to three original pharmaceutical products?'Gemzar' (active ingredient: gemcitabine) in 2020, the schizophrenia treatment 'Zyprexa' (active ingredient: olanzapine) in 2021, and Alimta in 2022?and has strengthened both its manufacturing competitiveness and profitability by producing them in-house. This approach also makes a significant contribution to stabilizing the pharmaceutical supply chain by ensuring continuity in prescriptions and a stable production and supply network.
According to data from the pharmaceutical market research firm IQVIA, Gemzar's annual prescription sales more than doubled from 14.3 billion won at the time of its acquisition in 2020 to 29.5 billion won last year. Zyprexa has continued steady growth since its acquisition in 2021, recording 16.7 billion won in prescription sales last year. Alimta grew by 28%, from 21 billion won in 2022 to 26.9 billion won in 2024.
Boryung is also expanding its contract development and manufacturing organization (CDMO) business based on these achievements. The cytotoxic anticancer injectable production facility at the Yesan Campus in Chungnam received EU-GMP certification from the European Medicines Agency (EMA) in 2023. Last year, Boryung signed a CDMO contract with the Taiwanese pharmaceutical company Lotus for the contract manufacturing of original anticancer drugs, and full-scale overseas supply is scheduled to begin in 2026.
Boryung is further advancing its LBA strategy by improving the formulations of original pharmaceuticals to enhance product value and patient convenience. Notably, the company developed a liquid formulation of Alimta, which was previously available only as a lyophilized powder, making it easier and safer to use. Previously, the drug had to be reconstituted immediately before administration, but with the new liquid injection form, preparation time is reduced and safety is improved, according to the company.
Earlier in 2023, Boryung also converted the anticancer drug Gemzar to a liquid formulation, and as of the first half of this year, this product accounted for over 70% of total Gemzar sales, indicating its rapid acceptance in the market.
Kim Jungkyun, CEO of Boryung, stated, "Boryung's LBA strategy is a growth strategy that goes beyond simple product acquisition to create new added value by leveraging our manufacturing infrastructure and research and development (R&D) capabilities. We will continue to actively secure global original products, internalize them, and supply them globally, thereby fulfilling our mission to become a company essential to human health."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.