First Observation of the Intermediate Step in Carbonic Anhydrase Reaction
Key Factors in the Enzyme Process Identified
Potential to Aid Drug Development and Biomimetic Catalyst Design
Published in Nature Communications
The reaction process of a biomolecular enzyme operating at the rapid speed of one million times per second has been captured using "molecular movie technology."
Similar to how a film is made by connecting individual frames, this technology freezes and photographs each step of the enzyme's reaction and then reconstructs them in sequence.
This research newly revealed the factors that determine enzyme activity, which is expected to aid in drug development and the design of biomimetic catalysts.
The research team led by Professor Chaeun Kim of the Department of Physics at UNIST succeeded in tracking, at the atomic level, the reaction in which carbonic anhydrase II converts carbon dioxide into carbonic acid.
 Research team, Professor Chaeun Kim (left) and Dr. Jingyun Kim (first author). Provided by UNIST
Research team, Professor Chaeun Kim (left) and Dr. Jingyun Kim (first author). Provided by UNIST
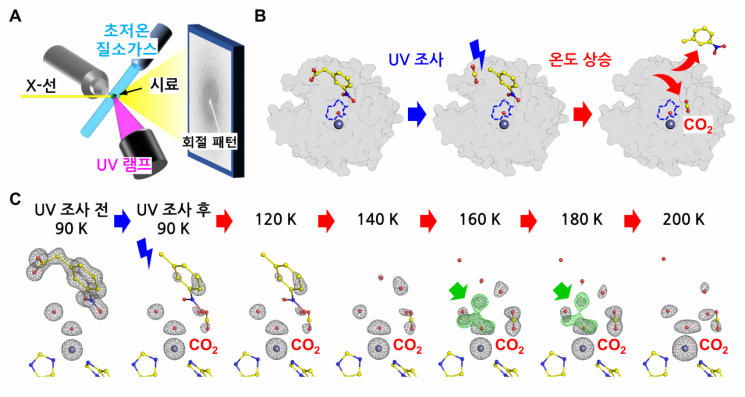
Carbonic anhydrase II is a protein catalyst that converts carbon dioxide into carbonate ions, which dissolve well in water. In this catalytic molecule's active site, carbon dioxide binds and is then converted into carbonate before being released. Because this reaction occurs more than one million times per second, it has long been considered virtually impossible to observe the intermediate steps of the process.
The research team captured the entire reaction process using their self-developed molecular movie technology and discovered that as water molecules rearrange and new water enters the active site, carbonate ions are rapidly released. This means that the rearrangement and replacement of water molecules during the reaction is a key factor determining the rate at which the product is released.
Molecular movie technology involves artificially halting the enzyme reaction by lowering the temperature, then continuously photographing the fixed structures with X-rays to reconstruct them in chronological order. The team crystallized the enzyme at -183°C and then introduced a photolabile substrate (3NPA).
This substrate instantly supplies carbon dioxide to the enzyme's active site upon exposure to ultraviolet light. By gradually raising the temperature to -73°C and photographing the structures at each stage, the team used this X-ray structural data to reconstruct the entire enzyme reaction process like a "molecular slow-motion movie."
Dr. Jingyun Kim, the first author, explained, "We were able to directly observe, at the atomic level, the intermediate state of the reaction that appears only in the temperature range from -113°C to -93°C. This is the first time in the world that such a fleeting intermediate step in an enzyme reaction has been structurally captured."
Professor Chaeun Kim expressed optimism, saying, "The newly discovered principle can be applied not only to protein engineering and drug development, but also to the design of biomimetic catalysts that precisely control water molecules."
The research results were published online in the world-renowned journal Nature Communications on May 12.
This research was supported by the Samsung Science and Technology Foundation and the National Research Foundation of Korea under the Ministry of Science and ICT, among others.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.