Water electrolysis has attracted attention as an eco-friendly method for producing hydrogen by splitting water using electricity. In particular, cation exchange membrane water electrolysis is considered a 'next-generation hydrogen production technology' because it can produce high-purity hydrogen at high pressure. However, this technology faces limitations in commercialization due to its heavy reliance on expensive precious metal catalysts and coatings. A new solution to overcome these technical and economic barriers has now been proposed in South Korea.
KAIST announced on June 11 that a research team led by Professor Heetak Kim from the Department of Chemical and Biomolecular Engineering, in collaboration with Dr. Kisu Do of the Korea Institute of Energy Research, has developed a next-generation water electrolysis technology that achieves high performance without the need for costly platinum (Pt) coatings.
 (Front row from left) Jisoo Park, PhD candidate; Heetak Kim, Professor (Back row from left) Kyunghwa Seok, PhD candidate; Kisu Do, PhD; Euntaek Oh, PhD candidate. Provided by KAIST
(Front row from left) Jisoo Park, PhD candidate; Heetak Kim, Professor (Back row from left) Kyunghwa Seok, PhD candidate; Kisu Do, PhD; Euntaek Oh, PhD candidate. Provided by KAIST
The joint research team was the first in the world to prove that the main reason 'iridium oxide (IrOx)', a highly active catalyst for water electrolysis electrodes, fails to deliver optimal performance is due to inefficient electron transfer. They also demonstrated that simply adjusting the particle size of the catalyst can maximize performance.
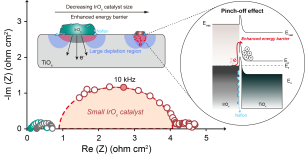
First, the team discovered that iridium oxide catalysts cannot achieve high performance without platinum coating because of electron transfer resistance that arises between the catalyst, the ion conductor (hereafter referred to as the ionomer)?a key component used in water electrolysis electrodes?and the titanium (Ti) substrate.
The research further identified that the 'pinch-off' phenomenon, in which the electron pathway is blocked between the catalyst, ionomer, and titanium substrate, is the primary cause of reduced conductivity.
The ionomer, which has properties close to those of an electronic insulator, can impede electron flow when it surrounds catalyst particles. Furthermore, when the ionomer comes into contact with the titanium substrate, an electron barrier forms on the surface oxide layer of the titanium, increasing resistance.
 Schematic diagram of the electron transfer resistance process at the catalyst layer diffusion layer interface. Provided by KAIST
Schematic diagram of the electron transfer resistance process at the catalyst layer diffusion layer interface. Provided by KAIST
To address this, the joint research team fabricated and compared catalysts of various particle sizes and conducted single-cell tests as well as multiphysics simulations. They demonstrated that when iridium oxide particles larger than 20 nm (nanometers) are used as catalyst particles, the ionomer-mixed regions are reduced, thereby securing electron pathways and restoring conductivity.
In addition, by precisely designing the interface structure, the team succeeded in optimizing the interface to ensure both reactivity and electron transfer. This showed that the trade-off between catalyst activity and conductivity, previously considered inevitable, can be overcome through precise interface engineering.
The joint research team believes that this achievement will serve as a significant milestone for the commercialization of cation exchange membrane water electrolysis systems, enabling high efficiency while drastically reducing the use of precious metals in high-performance catalyst development and future water electrolysis processes.
This research, with Jisoo Park, a PhD candidate in the Department of Chemical and Biomolecular Engineering at KAIST, as the first author, was published on June 7 in the international journal Energy & Environmental Science, which covers the fields of energy and the environment.
Professor Heetak Kim stated, "This study presents a new interface design strategy that resolves the interfacial conductivity bottleneck in high-performance water electrolysis technology. We hope this will bring us one step closer to realizing a hydrogen economy by enabling high performance in water electrolysis without the use of expensive materials such as platinum."
This research was supported by the Core Technology Development Project for New and Renewable Energy of the Ministry of Trade, Industry and Energy.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.











