Gwangju Institute of Science and Technology (GIST) announced on May 27 that the research team led by Professor Jaeyoung Lee (Director of the Future Research Center for Chemical Energy Storage and Conversion Processes) in the Department of Environmental and Energy Engineering has developed an electrochemical conversion technology capable of producing the high-value compound "allyl alcohol" from carbon dioxide at a world-leading "partial high current density."
This technology utilizes a membrane-electrode assembly with no gap between the electrodes, consisting of a self-developed copper phosphide (CuP2) reduction electrode catalyst and a nickel-iron (NiFe) oxidation electrode catalyst. By selectively converting carbon dioxide (which has one carbon atom) into the high-value multicarbon compound (C3+) allyl alcohol (which has three or more carbon atoms), the technology is expected to present a new possibility for economically viable electrochemical carbon capture and utilization (e-CCUs).
Allyl alcohol is a highly useful substance with a structure that includes both an allyl group with a double bond and a hydroxyl group, making it applicable in a wide range of chemical reactions. In particular, it is an essential raw material for synthesizing polymer compounds in various industries, including plastics, adhesives, disinfectants, and fragrances, and thus holds significant industrial value.
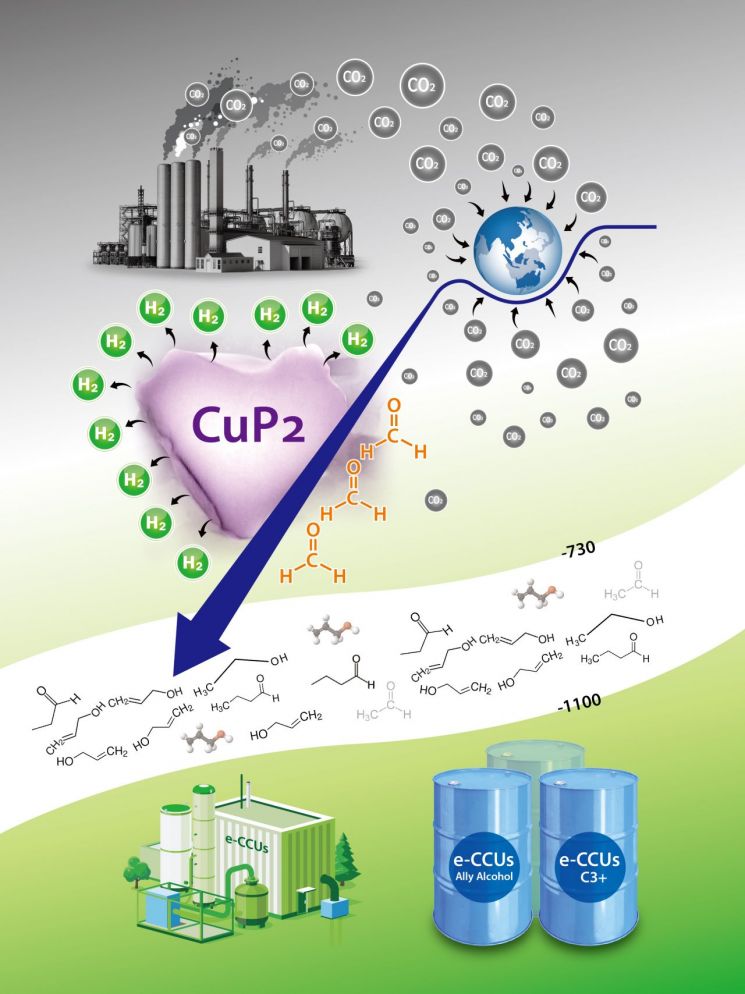 e-CCUs technology enabling C3+ multicarbon compound production from carbon dioxide using copper CuP2 electrode catalysts.
e-CCUs technology enabling C3+ multicarbon compound production from carbon dioxide using copper CuP2 electrode catalysts.
Electrochemical reduction technology for carbon dioxide is a core technology for the carbon-neutral era, as it can convert carbon dioxide?the main cause of global warming?into useful substances. However, selectively producing high-value compounds with three or more carbon atoms, such as allyl alcohol, has been highly limited due to Faradaic efficiencies of less than 15%, complex reaction pathways, and low stability of intermediates.
In particular, the production of liquid high-value compounds is known to be extremely challenging, as forming carbon-carbon bonds is difficult and the stability of reaction intermediates is low.
However, the technology developed in this study achieved a Faradaic efficiency of 66.9%, which is approximately four times higher than the previous best technology. This high efficiency demonstrates the catalyst’s outstanding selectivity, as it minimizes the formation of unnecessary byproducts and enables the selective production of the desired substance.
This research revealed a new reaction pathway in which a carbon-carbon (C?C) bond is formed during the conversion of the intermediate formate (HCOOad)* to formaldehyde (HCOad), rather than the widely known "carbon monoxide-mediated pathway." This reaction mechanism overturns conventional understanding and significantly increases the commercial value of the process, as most of the resulting products are liquid compounds that are easy to store and transport.
Professor Jaeyoung Lee stated, "The carbon dioxide conversion process technology we have developed can serve as a breakthrough, offering a new business direction for the coal, petrochemical, and steel industries, which are facing increasing pressure from carbon dioxide emissions." He added, "Through a scale-up, or large-scale scientific and technological approach, we expect this to become an important stepping stone toward a carbon-neutral era."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.












