Mentioning Neuralink's 'Brain Chip Implant' Surgery
"Robots Used Because Humans Can't Do It"... Threads Are Extremely Thin
Neuralink's Corporate Value Rises Ahead of $500 Million Fundraising
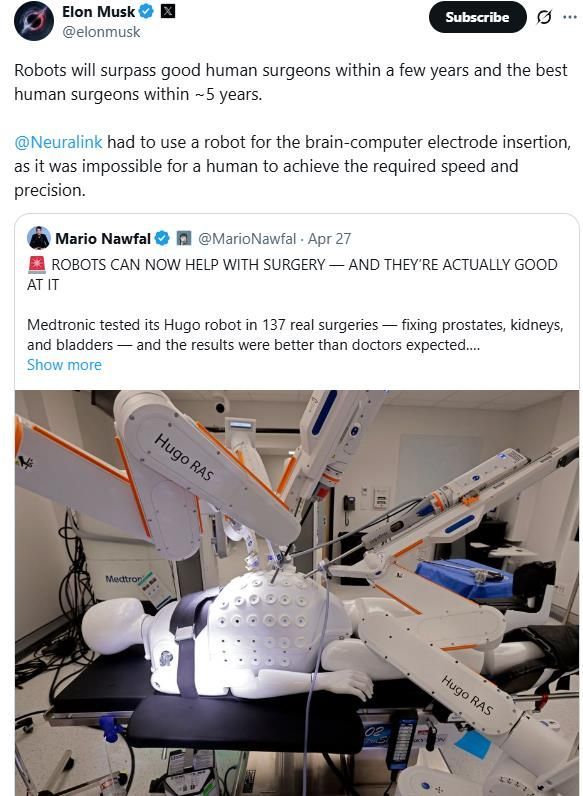
Elon Musk, CEO of Tesla, predicted on April 28 (local time) that surgical robot technology is advancing rapidly and will surpass human doctors within five years. He raised expectations for robot doctors by referencing a case of brain implant surgery performed by Neuralink, a neuroscience startup in which he is a major shareholder.
On the previous day, CEO Musk claimed in a post on X (formerly Twitter) that "robots will surpass excellent human surgeons within a few years and will outperform the best human surgeons within five years." He posted this statement while sharing a user's post on X, which said that a surgical robot product had achieved better results than doctors had anticipated in over 100 actual surgical trials.
He went on to mention Neuralink, the neuroscience startup he founded and operates, stating, "Neuralink had to use robots to insert brain-computer electrodes. It was impossible for humans to achieve the speed and precision required for this surgery."
According to the Neuralink website, "The threads used in our implants are so fine that they cannot be inserted by human hands. Our surgical robot is designed to insert these threads exactly where needed in a stable and efficient manner."
Additionally, CEO Musk shared on X the case of Bradford Smith, a patient with amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease) who is paralyzed and successfully communicated using a Neuralink chip implanted in his brain. Musk introduced this patient as the third paralyzed person to receive a Neuralink chip implant, and the first ALS patient. On the same day, Smith himself posted on X, saying, "I am typing this with my brain right now. This is my primary means of communication," and expressed his gratitude to CEO Musk.
Since last year, Neuralink has been conducting experiments in which a brain-computer interface (BCI) device, which remotely connects the human brain and computers, is implanted in the brains of paralyzed patients, enabling them to control various devices through the BCI. Initially, Neuralink failed to obtain clinical trial approval from the U.S. Food and Drug Administration (FDA) due to safety concerns, but later received approval and is currently conducting clinical trials. Neuralink plans to implant BCI devices in an additional 20 to 30 people this year.
It is known that Neuralink plans to conduct a Series E funding round this year. In April, Bloomberg News in the United States, citing anonymous sources, reported that Neuralink had begun discussions with potential investors to raise about $500 million (719.2 billion won) based on a pre-money valuation of $8.5 billion (about 12.13 trillion won). The last external funding round was in August 2023, when Neuralink succeeded in raising $280 million (about 366.2 billion won at the time) in a Series D round.
If the pre-money valuation is recognized by the market, the company's corporate value will rise significantly. According to financial market information provider PitchBook, Neuralink was valued at $3.5 billion as of November 2023. If the $8.5 billion valuation is confirmed in this funding round, the company's value will have increased by $5 billion in about a year and a half. While Tesla's stock value has recently plummeted due to tariff policies, the sharp rise in the value of unlisted stocks, including Neuralink, has offset the decline in CEO Musk's net worth. As of the close on April 28, Tesla shares were up 0.33% from the previous session at $285.88. This is 41% lower than the all-time high of $488.54 recorded on December 15 last year.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.