Strategy to Suppress Oxygen Gas Generation in Over-Lithiated Cathode Materials Proposed by UNIST and International Research Team
Fundamental Inhibition of Oxygen Oxidation via Transition Metal Substitution
...Published in Science Advances
A solution has been found for the problem of gas generation in next-generation long-range batteries.
The research team led by Professor Lee Hyunwook from the Department of Energy and Chemical Engineering at UNIST has identified the cause of oxygen evolution in over-lithiated cathode materials and proposed a material design principle to address this issue.
 Hyunwook Lee Professor.
Hyunwook Lee Professor.
Over-lithiated materials are theoretically capable of storing 30-70% more energy in batteries compared to conventional materials through high-voltage charging above 4.5V.
In terms of electric vehicle driving range, this means a single charge could allow travel of up to 1,000 km. However, during actual high-voltage charging, oxygen (O-2) trapped inside the material is oxidized and released as a gas (O2), increasing the risk of explosion.
The research team analyzed that partial structural deformation occurs near 4.25V as oxygen is oxidized, leading to the release of oxygen gas. They proposed an electrode material design method that fundamentally prevents this oxygen oxidation. The strategy involves substituting some of the transition metals in the over-lithiated material with transition metals of lower electronegativity.
Due to the difference in electronegativity between the two metal elements, electrons gather around the element with higher electronegativity, increasing the number of available electrons for the transition metal, which in turn prevents oxygen oxidation. Conversely, if the transition metal lacks available electrons, oxygen donates electrons instead, becomes oxidized, and is released as a gas.
Kim Minho, the first author and a Ph.D. from UNIST (currently a postdoctoral researcher at UCLA), explained, "Previous studies focused on stabilizing oxidized oxygen to prevent its release as a gas, but this study is distinguished by focusing on preventing the oxidation of oxygen itself."
 Minho Kim, PhD (first author).
Minho Kim, PhD (first author).
This change in electron density also induces a higher charging voltage, enabling higher energy density. Since energy density is proportional to the number of available electrons and the charging voltage, substituting transition metals allows more energy to be stored per unit weight of the battery. This is similar to how more energy can be stored when a dam has more water and a greater drop height.
The research team experimentally confirmed the oxygen oxidation suppression effect of the transition metal substitution strategy. Accelerator-based X-ray analysis showed that substituting some of the ruthenium with nickel significantly reduced the generation of oxygen gas. Additionally, density functional theory (DFT) calculations theoretically demonstrated the occurrence of charge redistribution.
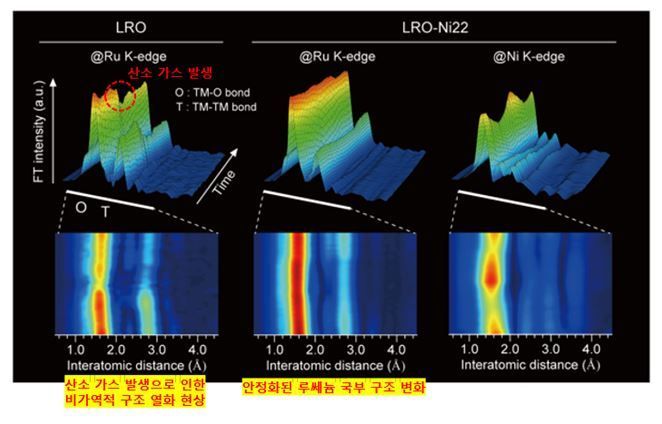 Real-Time Analysis of Local Structural Changes during Charge-Discharge of Cathode Materials Stabilized by Transition Metal Substitution
Real-Time Analysis of Local Structural Changes during Charge-Discharge of Cathode Materials Stabilized by Transition Metal Substitution
This research was a collaboration between Professor Seo Donghwa at KAIST, Chung-Ang University, Pohang Accelerator Laboratory, Professor Yuzhang Li at UCLA, UC Berkeley, and Lawrence Berkeley National Laboratory.
Accelerator-based X-ray analysis was conducted by Professor Jang Haesung of Chung-Ang University (co-first author), and DFT theoretical calculations were led by Dr. Lee Eunryeol of Lawrence Berkeley National Laboratory (co-first author).
Professor Lee Hyunwook stated, "By systematizing the technology through various experiments and theoretical analyses, we have provided direction for material development to cathode researchers," adding, "This will help in developing explosion-free, long-range battery materials with higher energy density."
This research was supported by the International Cooperation Program for Core Technology Development of the National Research Foundation of Korea, and the results were published online on February 19 in 'Science Advances,' a sister journal of the world-renowned journal 'Science' published by the American Association for the Advancement of Science (AAAS).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.











