MFDS: "Consumers Misled by Medical Procedure-Related Claims"
The Ministry of Food and Drug Safety announced on the 13th that it inspected 200 cosmetic sales posts distributed and sold online claiming effects such as 'cell regeneration,' 'anti-inflammatory,' and 'muscle relaxation,' and detected 144 cases of false and exaggerated advertising violating the 'Cosmetics Act.'
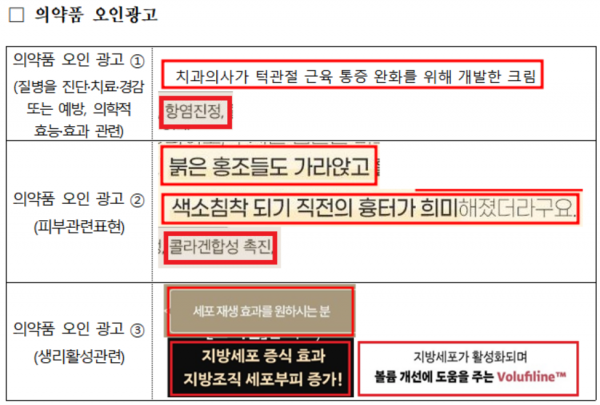 Types of Violations in Cosmetic Labeling and Advertising and Examples of Advertising Phrases. Provided by the Ministry of Food and Drug Safety
Types of Violations in Cosmetic Labeling and Advertising and Examples of Advertising Phrases. Provided by the Ministry of Food and Drug Safety
The detected advertisements include ▲83 cases (57.6%) of advertisements claiming pharmaceutical efficacy and effects, which may cause cosmetics to be mistakenly recognized as pharmaceuticals ▲39 cases (15.3%) of advertisements that deceive consumers or may cause consumers to misunderstand by presenting false information ▲22 cases (15.3%) of advertisements mistaken for functional cosmetics or differing from the functional cosmetics review content.
These products claimed medically unverified efficacy and effects such as 'cell regeneration,' 'adipocyte proliferation,' 'anti-inflammatory,' and 'muscle relaxation,' or used advertisements that could be misunderstood or misrecognized by consumers with false claims such as 'stem cells,' 'topical Botox,' 'filler procedure effects,' or advertisements that could be mistaken for functional cosmetics such as 'double chin lifting improvement.'
The Ministry of Food and Drug Safety urged consumers to be cautious not to be misled by false and exaggerated advertisements using medical procedure-related expressions such as Botox and fillers when purchasing cosmetics, and requested companies to refer to the 'Cosmetics Labeling and Advertising Management Guidelines,' which provide precautions and prohibited expressions for cosmetic labeling and advertising, to ensure proper labeling and advertising. Terms such as 'Botox,' 'filler,' 'fat volume generation,' and 'muscle relaxation' are prohibited expressions in cosmetic labeling and advertising.
Among the 144 detected false and exaggerated advertisements, the Ministry requested the Korea Communications Standards Commission and others to block 38 sales posts advertised by responsible cosmetic sellers, and plans to conduct on-site inspections and administrative actions through the local Food and Drug Safety Offices. Additionally, 25 of these cases involved general sales companies violating the Cosmetics Act, and investigations traced these to detect violations by responsible sellers.
An official from the Ministry of Food and Drug Safety stated, "We will continue to track and take action against illegal advertising activities by sellers, including advertisements by responsible sellers, to minimize consumer damage caused by illegal advertisements and to fundamentally resolve the root problems of false and exaggerated cosmetic advertising."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.











