Professor Shin Hyungjun's Team Reveals Quantum Phenomena Using a Triangle of Three Water Molecules
Molecular Rotation Accelerates Proton Tunneling... Published in Nano Letters
A research team has unveiled findings that analyze the shape changes of a triangle formed by three water molecules, revealing the quantum phenomena occurring within.
The team led by Professor Shin Hyungjun from the Department of Materials Science and Engineering at UNIST announced on the 10th that they have experimentally demonstrated that the collective rotational motion of water molecules enhances proton tunneling.
 Professor Shin Hyeongjun.
Professor Shin Hyeongjun.
Proton tunneling is a quantum mechanical phenomenon in which a proton (H+) passes directly through an energy barrier instead of overcoming it, influencing chemical reaction rates and the stability of biomolecules such as DNA.
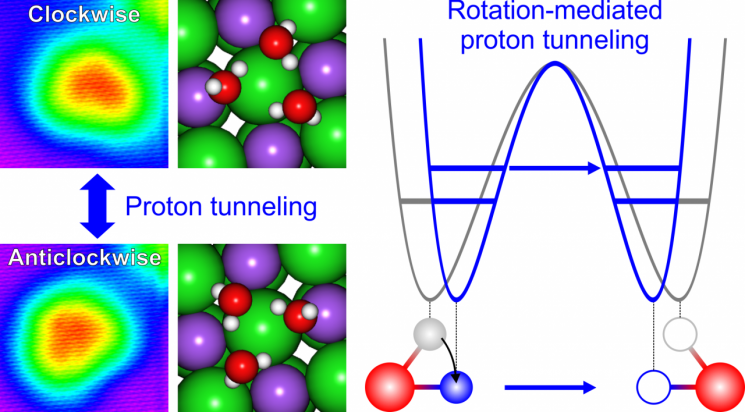
According to the research results, when the rotational motion of water molecules is activated, the distance between molecules is adjusted, increasing cooperativity and thereby promoting proton tunneling. With increased cooperativity, the protons (H+) of the three water molecules collectively penetrate the energy barrier.
The research team discovered this by forming a triangle with three water molecules and analyzing the changes in the shape of this triangle. To move the molecules one by one and arrange them into the desired shape, as well as to analyze the shape changes, they used an advanced analytical technique called scanning tunneling microscopy (STM).
The water molecules were arranged and fixed on a salt thin film, and maintained under ultra-high vacuum (10-11 Torr) and ultra-low temperatures (-268.75°C to -257.15°C) to prevent evaporation.
The triangle of water molecules placed on the salt thin film exhibited a distorted (isomeric) shape, leaning to the left or right, and the direction of distortion changed frequently.
This serves as observational evidence that proton tunneling occurs naturally even at low temperatures.
When a specific voltage was applied to the water molecule triangle using the probe of the scanning tunneling microscope in this state, the distorted triangle shifted to a shape closer to an equilateral triangle. This resulted from the voltage-activated rotational motion of the molecules adjusting the hydrogen bond length, which is the distance between water molecules. This structural change in the triangle enhanced molecular cooperativity and led to collective proton tunneling.
To verify this, the research team conducted additional experiments, including theoretical calculations and comparative analysis of tunneling rates between heavy water (D2O) and regular water (H2O), in addition to observing the shape of the water molecule triangle.
Yohan Kim and Heejun Han from UNIST participated as co-first authors in this study.
 Dr. Yohan Kim (Co-first author).
Dr. Yohan Kim (Co-first author).
 Heejoon Han PhD (co-first author).
Heejoon Han PhD (co-first author).
The researchers explained, "Water is a useful tool for experimentally analyzing the relationship between molecular cooperativity and proton tunneling, but such experiments have been challenging due to the strong hydrogen bonds between water molecules. We resolved this issue by developing a technique that allows us to isolate and analyze just three water molecules."
Corresponding author Professor Shin Hyungjun stated, "This study is significant in that it experimentally identifies the rotational motion and molecular cooperativity of water molecules as key factors that can regulate proton tunneling. These findings may offer new methods for controlling reactions in chemical processes, catalysis, and energy conversion."
The research results were published in the international journal Nano Letters on February 12. The study was supported by the Mid-career Researcher Program and the Institute for Basic Science.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.