Mechanism of Rapid Coronavirus Replication Unveiled
Discovery of Dual Functions in nsp13 Protein
Accelerated RNA Replication Explains High Contagiousness
New Insights for Vaccine and Therapeutic Development
Researchers at the Korea Advanced Institute of Science and Technology (KAIST) have uncovered the mechanism by which the replication process of the core enzyme protein (helicase) of the coronavirus is rapidly accelerated, leading to its contagiousness.
Severe Acute Respiratory Syndrome (SARS) and COVID-19 spread globally in a short period as pandemics, but the mechanism behind their rapid replication and transmission had not yet been elucidated. This study presents new possibilities for the development of virus vaccines and therapeutics.
 Yoo Jeong-min, Ph.D. in Biological Sciences at KAIST (left), Professor Lee Kwang-rok. Provided by KAIST
Yoo Jeong-min, Ph.D. in Biological Sciences at KAIST (left), Professor Lee Kwang-rok. Provided by KAIST
The research team led by Professor Kwang Rok Lee from the Department of Biological Sciences at KAIST announced on the 17th that the 'nsp13 protein,' a crucial enzyme for coronavirus proliferation, has two activities that work synergistically to promote the replication of the SARS coronavirus's genetic material, RNA.
The first activity of the nsp13 protein, corresponding to helicase activity, is an enzyme function that unwinds double-stranded nucleic acids such as DNA or RNA into single strands, facilitating replication and transcription processes. The second activity, RNA chaperone activity, is a protein function that assists in the correct folding and unfolding of nucleic acid structures, correcting misfolded RNA or enhancing stability, thereby aiding intracellular RNA metabolic processes.
For the coronavirus to spread rapidly, it is essential to quickly replicate its genetic material and produce the constituent proteins to assemble them.
Although it was previously unknown why the RNA replication of the genetic material in the first stage occurs faster than in other viruses, the research team discovered that the nsp13 protein accelerates the gene replication process through its conventional helicase activity and a newly identified chaperone activity.
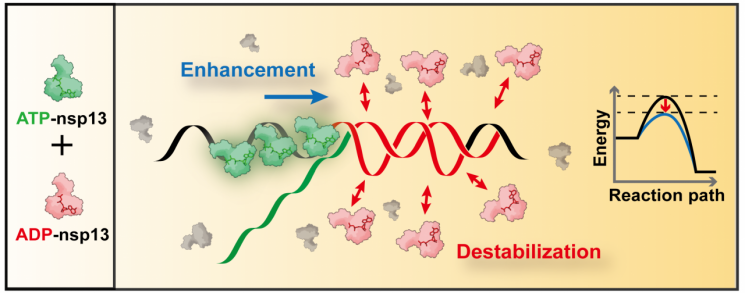 Schematic diagram of cooperative unfolding activation of the substrate of 'nsp 13' protein through two methods. Provided by Professor Kwangrok Lee's research team
Schematic diagram of cooperative unfolding activation of the substrate of 'nsp 13' protein through two methods. Provided by Professor Kwangrok Lee's research team
The nsp13 protein is genetically well conserved and is an important target for vaccines or infection treatments against various mutant coronaviruses, but a complete understanding of its exact mechanism of action was lacking.
The research team found that the nsp13 helicase uses the chemical energy released from the hydrolysis of ATP (adenosine triphosphate), which supplies energy for the enzyme's function, to unwind the twisted structure of RNA into single strands, producing ADP (adenosine diphosphate) as a byproduct. They also discovered that when the produced ADP recombines with nsp13, it activates the chaperone function, further destabilizing the RNA secondary structure.
In conclusion, they elucidated a novel mechanism in which helicase activity and chaperone activity cooperate spatiotemporally to promote RNA replication.
Professor Kwang Rok Lee explained, "This study is a new discovery showing that the representative nucleic acid-enzyme protein helicase exhibits chaperone-like activity through ADP," adding, "It broadens our understanding of the functional diversity of helicase and is expected to provide clues for developing effective therapeutics and vaccines to combat various mutations of the SARS coronavirus."
This research, with Dr. Jung Min Yoo as the first author, was published online on the 29th of last month in the world-renowned international journal Nucleic Acids Research (IF: 16.7, top 1.8% in the field of biochemistry and molecular biology).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.











