First-Ever Design Strategy for Anion-π Interaction in Mussel-Inspired Polymer Systems
Published in the Prestigious Journal PNAS, with Applications in Adhesives, Self-Assembly Systems, Catalysts, and Drug Delivery
The research team led by Professor Byungsoo Kim from the Department of Chemistry at Yonsei University, in collaboration with Professor Dongwook Lee's team at Ulsan National Institute of Science and Technology (UNIST), has identified that the 'anion-π interaction' plays a crucial role as a key principle in enhancing the cohesion of polymers.
 (From left) Professor Byungsoo Kim, Yonsei University; Professor Dongwook Lee, Ulsan National Institute of Science and Technology. Photo by UNIST
(From left) Professor Byungsoo Kim, Yonsei University; Professor Dongwook Lee, Ulsan National Institute of Science and Technology. Photo by UNIST
In this study, the team developed polymers based on epoxy monomers mimicking mussel foot proteins and experimentally demonstrated that anion-π interactions are a critical factor in strengthening polymer cohesion.
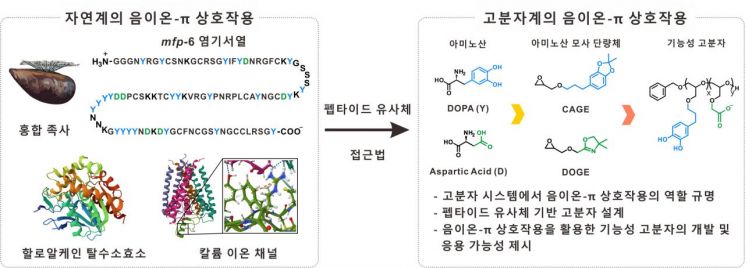
Anion-π interaction refers to a non-covalent bond occurring between an anionic molecule (bearing a negative charge) and the π electron system of an aromatic ring. Although this interaction plays an important role in biological systems such as enzyme catalysis and ion transport, actual research cases have been extremely rare. In particular, studies utilizing this interaction in synthetic polymers have been almost nonexistent.
Inspired by this, the research team focused on mussels, which possess strong adhesion in nature. Mussel foot proteins have strong bonding ability that allows them to firmly attach even in seawater. Analysis of the key components enabling this revealed that the structural features of DOPA (3,4-dihydroxyphenylalanine) and aspartic acid play an important role.
The team designed functional monomers mimicking these structural features and synthesized new polymers. Through this, they proposed a new polymer design approach that considers the complex intermolecular interactions observed in biological systems.
Specifically, the monomer mimicking DOPA provides the π electron system of the aromatic ring, while the monomer mimicking aspartic acid provides the anion, enabling anion-π interactions within the polymer. Additionally, the team quantitatively analyzed the cohesion of the polymers under various conditions using a Surface Forces Apparatus (SFA).
The researchers analyzed the difference in cohesion between neutral environments, where the polymer functional groups exist in an ionized state, and acidic environments, where they exist in a non-ionized state. As a result, in neutral environments, anion-π interactions acted as the main bonding force, significantly increasing polymer cohesion, whereas in acidic environments, hydrogen bonding played a major role, resulting in relatively weaker cohesion.
This study is the first experimental demonstration that anion-π interactions play a decisive role in enhancing cohesion in synthetic polymers. Based on this, new polymer design strategies are expected to be applied in various fields such as adhesives, self-assembly systems, catalysts, and drug delivery.
The research team stated, “Through this study, we have presented a method to apply the principle of anion-π interactions to polymer design,” adding, “This research not only expands the industrial application potential of synthetic polymers but also contributes to the academic advancement of polymer chemistry.”
This research was conducted as a mid-career researcher support project funded by the Ministry of Science and ICT and the National Research Foundation of Korea. The research results were published online on February 6, 2025, in the globally prestigious journal Proceedings of the National Academy of Sciences (PNAS).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.