Korean biosimilar (biopharmaceutical generic) companies are intensifying their efforts to target the United States, the world's largest market, again this year. With market expansion expected due to drug price reduction policies during the Donald Trump administration, the steady preparation of supply by our companies is anticipated to lead to growth in their scale.
According to the bio industry on the 7th, the top two domestic biosimilar companies, Celltrion and Samsung Bioepis, are expected to launch more than seven products and receive more than four approvals from the Food and Drug Administration (FDA) this year alone.
Based on this, Celltrion is expected to launch biosimilars of ▲ the autoimmune disease treatment 'Stelara' ▲ the ophthalmic disease treatment 'Eylea' ▲ the autoimmune disease treatment 'Actemra' ▲ the osteoporosis treatment 'Prolia' ▲ and the asthma and allergy treatment 'Xolair' this year.
The first FDA approval for a biosimilar this year also went to Celltrion. On the 31st of last month, Celltrion obtained FDA approval for 'Aptozma,' a biosimilar of the Swiss Roche autoimmune disease treatment 'Actemra.' Currently, Celltrion has also applied for FDA approval for the allergy treatment biosimilar 'Omriklo' of Xolair and the osteoporosis treatment biosimilar 'Stovoclo' of Prolia.
Samsung Bioepis is also pursuing FDA approval for the Prolia biosimilar 'SB16.' Additionally, it plans to launch the biosimilar 'Episcle' of the blood disease treatment Soliris and the biosimilar 'Fizchiba' of the autoimmune disease treatment Stelara, both of which have already received approval, within the first half of this year.
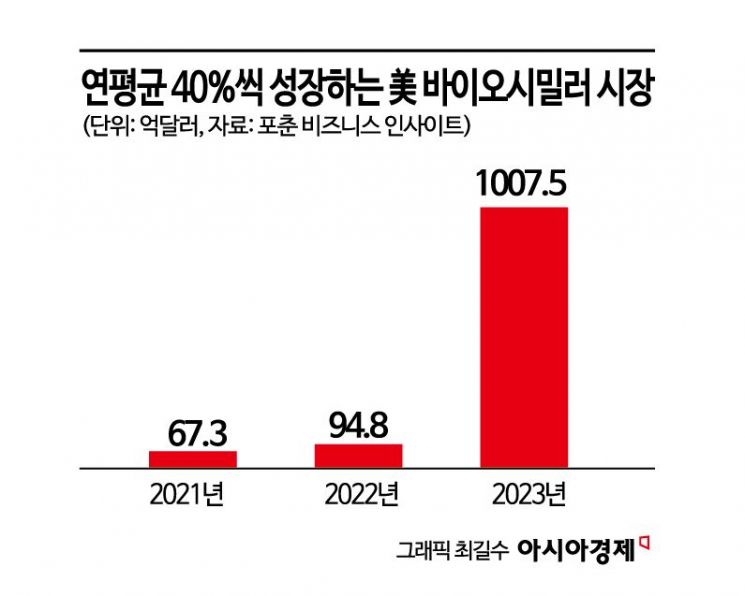
The United States is the world's largest biosimilar market. According to Fortune Business Insights, the U.S. biosimilar market was valued at $6.73 billion (approximately 9.7369 trillion KRW) in 2021, and is expected to grow from $9.48 billion (approximately 13.7156 trillion KRW) in 2022 to $100.75 billion (approximately 145.7651 trillion KRW) by 2029, showing a compound annual growth rate (CAGR) of 40.2% during the forecast period.
South Korea obtained the most FDA biosimilar approvals worldwide last year. According to the Korea Bio Association, the FDA approved a total of 18 biosimilars during this period, with Korea and the U.S. each having 4 approvals, Germany 3, India, Switzerland, and Iceland 2 each, and Taiwan 1. Including 'Imuldosa,' developed by Korea's Dong-A ST and approved by the U.S. 'Accord Biopharma' in October last year, Korea has 5 approvals and the U.S. 3, making Korea the leader. From 2015 to last year, the FDA approved a total of 63 biosimilars for 17 original drugs over 10 years, with Korean companies receiving 14 approvals, the second highest after the U.S. with 26.
Last year is notable for having the highest number of approvals since the FDA first approved biosimilars in 2015. It appears that the U.S. is taking a proactive stance on biosimilar approvals to reduce healthcare costs. Biosimilars are generally about 30% cheaper than the original products. Donald Trump, during his presidential campaign, pledged to promote the use of generics and biosimilars as part of drug price reduction policies, so the market is expected to grow further in the future.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.