GI Innovation's affiliate, GI Cell, announced on the 17th that it has received approval from the Ministry of Food and Drug Safety for the clinical trial application (IND) of the allogeneic natural killer (NK) cell therapy 'T.O.P NK (GIC-102)'. GI Cell plans to begin clinical trials in the first half of this year.
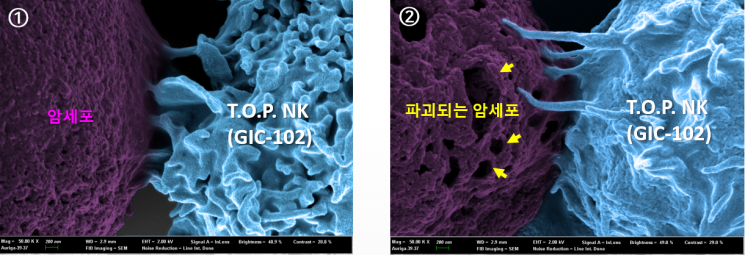 A photograph captured by an electron microscope showing the cancer cell-killing process of the T.O.P. NK cell therapy.
A photograph captured by an electron microscope showing the cancer cell-killing process of the T.O.P. NK cell therapy. [Photo by GI Cell]
This clinical trial is a domestic Phase 1 study to evaluate the safety and potential efficacy of T.O.P. NK in patients with advanced solid tumors. T.O.P. NK is a next-generation NK cell therapy with enhanced tumor-targeting ability and cancer cell killing efficiency. Using GI Cell's immune cell mass culture platform technology, 'Immune CellPure Expander,' it is possible to mass-produce high-purity cells.
GI Cell demonstrated the efficacy of T.O.P. NK against various solid tumors in non-clinical trials using humanized mice. In particular, consistent tumor growth inhibition was confirmed in mice implanted with various types of colon cancer cell lines.
In Phase 1 clinical trials, GI Cell plans to evaluate T.O.P. NK not only as a monotherapy but also in combination with GI Innovation's bispecific fusion protein 'GI-101.' GI-101, currently in Phase 1/2 clinical trials, is a fusion protein combining CD80, which targets immune cells, and an IL-2 mutant (IL-2v) that overcomes the limitations of conventional interleukin (IL)-2. It has shown efficacy in clinical trials involving patients with various solid tumors, including colon cancer.
Jang Myung-ho, Head of Clinical Strategy at GI Innovation, stated, “GI-101 specifically binds to receptors expressed on NK cells, promoting their proliferation and activation. Therefore, we expect that combination therapy with T.O.P. NK will demonstrate excellent synergy in various solid tumors, including colon cancer.” Hong Cheon-pyo, CEO of GI Cell, also said, “We have taken a step closer to our goal of providing new treatment options for cancer patients who are unresponsive to standard therapies. Since the combination therapy of T.O.P. NK and GI-101 shows outstanding in vivo persistence and efficacy, we will do our best to obtain product approval as quickly as possible through rapid clinical validation.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.














