Indian 'Death Cold Medicine'... 300 Children Dead
This Time, Using Indian Artificial Tears Leads to Pseudomonas Infection... Deaths Confirmed
[Asia Economy Reporter Yoon Seul-gi] Safety issues with Indian-made pharmaceuticals are repeatedly emerging. Starting with Gambia last year, 300 children in seven countries including Indonesia and Uzbekistan died in groups after taking Indian-made syrup cold medicine. Now, there has been an incident where people died or went blind after using artificial tears made by an Indian pharmaceutical company.
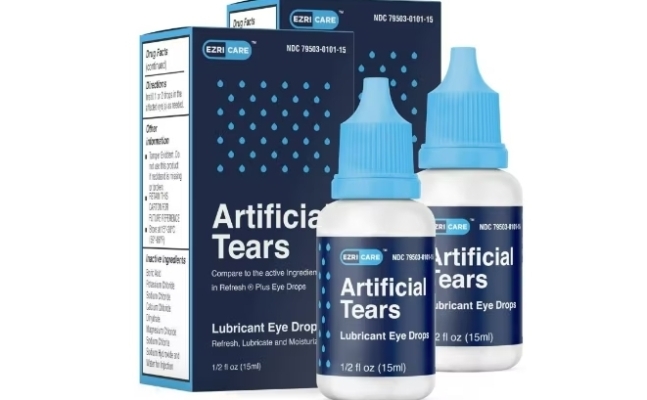
In the United States, there have been cases of people going blind or dying after using artificial tears. According to recent reports by the US public broadcaster NPR, from May last year to last month, 55 patients in 12 states including California, New York, and Florida were infected with Pseudomonas aeruginosa after using artificial tears made by an Indian pharmaceutical company. Among them, one person died and at least five went blind.
The problematic product is 'EzriCare' by the Indian pharmaceutical company Global Pharma. Currently, the US Food and Drug Administration (FDA) has decided to halt sales, and the manufacturer Global Pharma has initiated a recall of EzriCare products distributed nationwide in the US.
India is one of the world's largest pharmaceutical producers and is also called the 'pharmacy of the world,' but recently, pharmaceutical safety issues have repeatedly surfaced. According to a World Health Organization (WHO) announcement on the 23rd of last month, toxic substances were found mixed in children's cough medicines on the market, resulting in over 300 deaths so far in seven countries including Gambia, Indonesia, and Uzbekistan.
 The World Health Organization (WHO) has warned that eight types of syrup medicines from Indonesia contain harmful substances that can cause death. Photo by EPA·Yonhap News
The World Health Organization (WHO) has warned that eight types of syrup medicines from Indonesia contain harmful substances that can cause death. Photo by EPA·Yonhap News
The earlier cold medicine incident arose after 66 children in Gambia died from unknown causes after taking cough and cold syrup products from India's Maiden Pharmaceuticals. Most of the deceased children were aged between 5 months and 5 years, and the cause of death was acute kidney injury. The death toll later rose to over 70.
In December of the same year, 18 children in Uzbekistan died from viral acute respiratory infection symptoms such as influenza after taking syrup medicine from the Indian pharmaceutical company Marion Biotech. In Indonesia, there was also a mass death of infants after taking syrup-type cold medicine, with the number of deceased infants reaching around 200 as of January.
In a statement that day, WHO recommended banning the distribution of four syrup products containing harmful substances manufactured by Maiden Pharmaceuticals, stating that unacceptable levels of diethylene glycol and ethylene glycol were detected in the syrup medicines. These two raw materials are mainly used industrially as antifreeze in electronics such as air conditioners, refrigerators, and freezers, or as brake oil, but are also used in trace amounts in pharmaceutical manufacturing. They are inexpensive and are used as substitutes for glycerin in some low-cost medicines.
Meanwhile, it is known that the problematic Indian-made pharmaceuticals such as syrup-type cold medicines and artificial tears are not imported into South Korea.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.