Hanmi, Dong-A, GC, and Others
Facility Upgrades and Supply Contracts in Focus
[Asia Economy, reporter Lee Gwanjoo] Domestic pharmaceutical companies are increasingly entering the contract development and manufacturing organization (CDMO) business. This move is interpreted as an effort to find new sources of revenue in the post-COVID-19 era. The ability of new entrants to prove their competitiveness is expected to determine their success or failure in the global market.
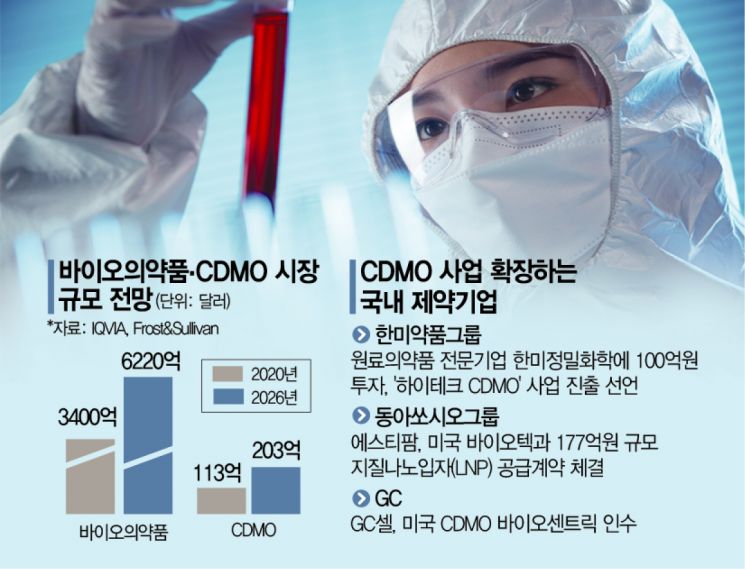
According to the pharmaceutical industry on May 6, Hanmi Fine Chemical, a subsidiary of Hanmi Pharmaceutical specializing in active pharmaceutical ingredients, has recently announced its entry into the 'high-tech CDMO' business. The company plans to provide CDMO services for advanced raw materials, including lipid nanoparticles (LNPs) used as key ingredients in messenger RNA (mRNA) vaccines, which gained attention during the COVID-19 pandemic, as well as nucleotides, polyethylene glycol (PEG) derivatives, and peptides, all of which are raw materials for biopharmaceuticals. To this end, Hanmi Fine Chemical has decided to invest a total of 10 billion won, which includes 1.6 billion won secured from a government project to expand COVID-19 vaccine and raw material production facilities last year, as well as approximately 8 billion won of its own funds, to upgrade its facilities.
Hanmi believes it has sufficient competitiveness in the CDMO business. Hanmi Fine Chemical has experience participating in Hanmi Pharmaceutical's development of bio and anticancer drugs and has established a foundation for mass production of high-purity drug substances. Currently, Hanmi Fine Chemical is carrying out preclinical and clinical CDMO projects worth about 10 billion won with more than 10 domestic and international companies. The company also plans to participate in various international exhibitions, such as the 'Bio International Convention' to be held in the United States next month, to expand partnerships in the second half of the year. A Hanmi Pharmaceutical Group representative stated, "Expanding our CDMO business will be a core driver of future growth."
ST Pharm, an active pharmaceutical ingredient specialist of Dong-A Socio Group (which includes Dong-A Pharmaceutical), has also signed a contract with a US biotech company to supply 17.7 billion won worth of lipids, a key ingredient in LNP composition, thereby launching its global mRNA CDMO business in earnest. ST Pharm highlights its ability to mass-produce 3 tons of ionizable lipids and 1 ton of PEG lipids annually at its GMP-certified facilities as a competitive strength.
Furthermore, the company has secured mRNA platform technology by developing its own capping technology and discovering next-generation LNP candidate substances, for which it has completed domestic patent applications. This is seen as a competitive advantage in the CDMO business. Including this supply contract, ST Pharm has signed mRNA-related contracts worth 24.8 billion won to date. The company has also discussed joint development of mRNA vaccines and CDMO services with seven companies, including two global pharmaceutical firms. ST Pharm plans to accelerate its global market expansion by participating in the 'TIDES USA' international conference to be held in Boston, USA, from May 9 to 12.
GC Cell, which was established through the merger of GC Labcell and GC Cell in November last year, is acquiring a 100% stake in BioCentriq, a US-based CDMO company. BioCentriq is recognized as a world-class CDMO specializing in cell therapies, gene therapies, and viral vectors. GC Cell believes that this acquisition will enable it to accelerate its entry into the US CDMO market. A GC Cell representative said, "This investment is aimed at rapidly scaling up our CDMO capabilities," adding, "We are also planning to expand additional facilities in North America."
The reason domestic pharmaceutical companies are entering the CDMO business is seen as an effort to secure sustainable growth momentum in the post-COVID-19 era. An industry official commented, "The biggest issue in the pharmaceutical industry recently has been which areas to expand into in the post-COVID-19 era," and added, "CDMO business expansion will continue, especially among companies with strong capital and technology. However, as competition intensifies, proving technological capability and production capacity will be key points."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.